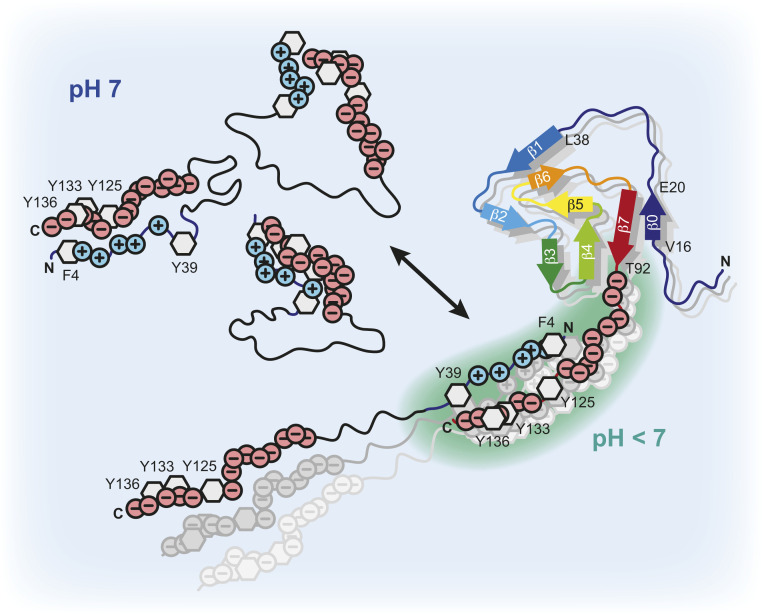
Fig. 5.
Scheme of α-Syn fibril interactions. Transient long-range intramolecular contacts between N- and C-terminal residues in soluble α-Syn yield partially collapsed intrinsically disordered states of the protein and occlude the central aggregation-prone NAC region. The highly negatively charged C terminus of the α-Syn fibrils lowers the local pH compared to the bulk. Intermolecular α-Syn-fibril interactions between the positively charged N-terminal segment of α-Syn and the negatively charged flexible C-terminal tails of the fibrils unfold the loosely packed α-Syn structures and dynamically align α-Syn molecules at high local concentrations on the fibril surface. This results in an exposure of the NAC region and triggers protein aggregation via secondary nucleation.