Scheme 1. Twelve nucleobase amino acid monomers were synthesized (A) and incorporated into peptides by SPPS (B).
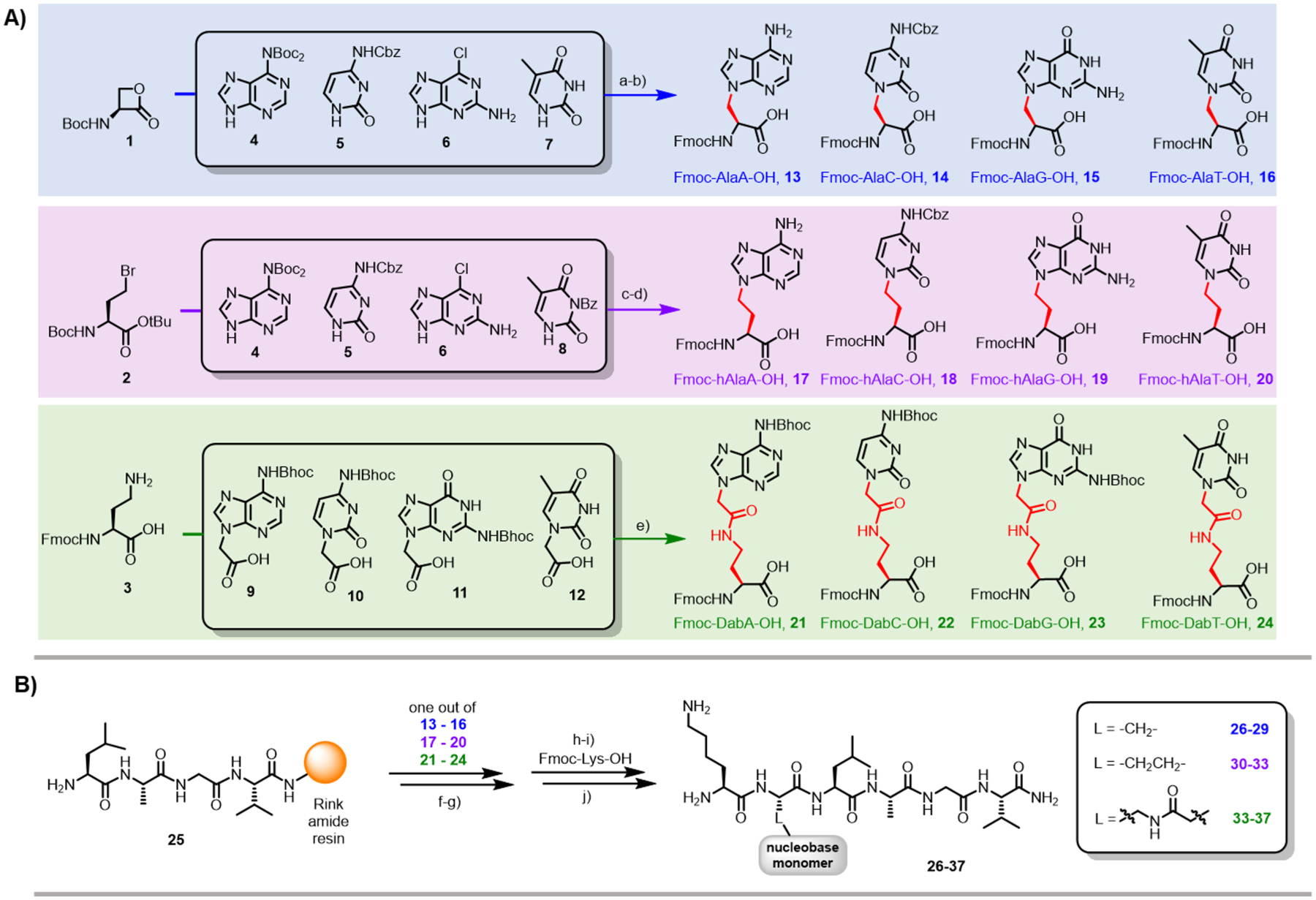
Reagents and conditions: a) DBU, DMSO, −78 °C, 2 h, 38% for 4, 39% for 5, 63% for 6, 29% for 7; b) TFA/CH2Cl2 (3:1), 90 min, r.t.; (for 6: TFA/H2O (3:1), 72 h, r.t.); then: Na2CO3, FmocOSu, H2O/1,4-dioxane, 16 h, 54% for 13, 61% for 14, 74% for 15, 63% for 16; c) K2CO3, Cs2CO3, DMF, 50 °C, 16 h, 58% for 4, 60% for 5, 70% for 6, 80% for 8; d) TFA/CH2Cl2 (3:1), 90 min, r.t.; (for 6: HCl (1 M), 2 h, 90 °C; for 8: HBr/CH3COOH, 1 h, r.t.); then: Na2CO3, FmocOSu, H2O/1,4-dioxane, 16 h, 73% for 17, 42% for 18, 42% for 19, 70% for 20; e) TSTU, DMF, DIEA, 2 h, 28% for 21, 22% for 22, 25% for 23, 28% for 24; f) PyAOP, DIEA, DMF, 20 min, r.t.; g) piperidine, DMF, 5 min, r.t.; h) HATU, DIEA, DMF, 10 min, r.t.; i) piperidine, DMF, 5 min, r.t.; j) TFA, TMSOTf, EDT, thioanisole, cresol, 1 h, r.t. Abbreviations: DBU = 1,8-Diazabicyclo[5.4.0]undec-7-ene, DMSO = dimethyl sulfoxide, TFA = trifluoroacetic acid, r.t. = room temperature, FmocOSu = N-(9H-fluoren-9-ylmethoxycarbonyloxy)succinimide, DMF = N,N-dimethylformamide, TSTU = N,N,N′,N′-tetramethyl-O-(N-succinimidyl)uronium tetrafluoroborate, DIEA = N,N-diisopropylethylamine, PyAOP = (7-azabenzotriazol-1-yloxy)trispyrrolidinophosphonium hexafluorophosphate, HATU = 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxid hexafluorophosphate, TMSOTf = trimethylsilyl trifluoromethanesulfonate, EDT = 1,2-ethanedithiol.
