Abstract
Background: Central sleep apnea (CSA) is common among patients with heart failure and has been strongly linked to adverse outcomes. However, progress toward improving outcomes for such patients has been limited. The purpose of this official statement from the American Thoracic Society is to identify key areas to prioritize for future research regarding CSA in heart failure.
Methods: An international multidisciplinary group with expertise in sleep medicine, pulmonary medicine, heart failure, clinical research, and health outcomes was convened. The group met at the American Thoracic Society 2019 International Conference to determine research priority areas. A statement summarizing the findings of the group was subsequently authored using input from all members.
Results: The workgroup identified 11 specific research priorities in several key areas: 1) control of breathing and pathophysiology leading to CSA, 2) variability across individuals and over time, 3) techniques to examine CSA pathogenesis and outcomes, 4) impact of device and pharmacological treatment, and 5) implementing CSA treatment for all individuals
Conclusions: Advancing care for patients with CSA in the context of heart failure will require progress in the arenas of translational (basic through clinical), epidemiological, and patient-centered outcome research. Given the increasing prevalence of heart failure and its associated substantial burden to individuals, society, and the healthcare system, targeted research to improve knowledge of CSA pathogenesis and treatment is a priority.
Keywords: sleep apnea, heart failure, respiration
Contents
Overview
Overall Conclusions
Research Priorities
Introduction
Methods
Findings
Key Area: Control of Breathing and Pathophysiology Leading to CSA
Key Area: Variability in CSA across Individuals and over Time
Key Area: Techniques to Examine CSA Pathogenesis and Outcomes
Key Area: Impact of Device and Pharmacological Treatment
Key Area: Implementing CSA Treatments for All Individuals
Conclusions
Overview
Central sleep apnea (CSA) is common among patients with heart failure and is strongly and independently associated with poor health and well-being outcomes. To date, there are no therapies directed at CSA that consistently improve outcomes in this population. This American Thoracic Society (ATS) statement summarizes current literature, identifies gaps in knowledge, and outlines a path forward for research with specific recommendations.
Overall Conclusions
-
1.
Substantial advances in knowledge have been made regarding the neurobiology of the control of breathing, including in relation to CSA. Additional work will help to identify novel potential therapeutic targets.
-
2.
There is a need to advance knowledge of the differences in CSA across individuals in terms of both pathogenesis and clinical outcomes. This work will benefit from looking beyond the apnea–hypopnea index (AHI).
-
3.
Within individuals, CSA can change substantially over time. Factors influencing such changes and their relevance are poorly understood.
-
4.
There are several available and emerging treatments for CSA that show promise. These may be best optimized using an individualized strategy that incorporates the heterogeneity of CSA pathophysiology and its consequences, together with consideration of potential approaches to health disparities.
Research Priorities
-
1.
Advance basic and translational research regarding control of breathing.
-
2.
Determine mechanisms that mediate breathing instability in CSA.
-
3.
Examine CSA in diverse groups and across the adult life span.
-
4.
Evaluate changes in CSA over time and the relationship with heart failure status.
-
5.
Develop and validate tools to better characterize CSA (i.e., beyond the AHI).
-
6.
Determine how sleep apnea physiology might contribute to—or protect against—heart failure progression.
-
7.
Determine the impact of established and emerging heart failure therapies on CSA.
-
8.
Clarify the role of positive airway pressure (PAP) therapy for CSA in those with heart failure.
-
9.
Establish the utility of supplemental oxygen, inspired CO2, and pharmacotherapy for treatment for CSA.
-
10.
Determine factors influencing adherence to CSA treatments.
-
11.
Characterize health disparities related to CSA across populations.
Introduction
CSA is common among patients with heart failure, particularly in those with heart failure with reduced ejection fraction (HFrEF), but is also observed in those with preserved ejection fraction (1–3). The presence of CSA is associated with important prognostic implications as a marker of heart failure severity (4, 5). Furthermore, CSA is associated with sleep disruption, oxygen desaturation, and increases in sympathetic activity and thus may itself directly adversely impact patient outcomes. Despite sharing these manifestations with obstructive sleep apnea (OSA), CSA has been the focus of comparatively little research. While OSA is characterized by upper airway obstruction (defined by obstructive apneas and/or hypopneas), CSA is distinguished by recurrent changes in ventilation and ventilatory drive leading to central events. Accordingly, a more limited understanding of CSA pathophysiology and its clinical implications has resulted in a much smaller evidence base to inform clinical care for patients with CSA.
The aim of this statement is to outline a path forward for research regarding CSA as it pertains to those with heart failure, with the ultimate goal of improving outcomes for these individuals. Although the focus of this research priority statement is CSA in those with heart failure, we expect that progress will be broadly relevant to other forms of CSA, including idiopathic CSA and opioid-related CSA, among others. For each section, a key area for research is followed by a brief summary of the current understanding of CSA and the rationale for additional investigation. For additional background on CSA, we refer the reader to the recent European Respiratory Society (ERS) task force report (6). For the most recent clinical management and treatment guidelines regarding CSA in heart failure, we refer the reader to the American Academy of Sleep Medicine (AASM) clinical practice guidelines (7).
Methods
This research statement was developed according to the guidelines specified by the ATS. Potential conflicts of interest were disclosed and managed in accordance with the policies and procedures of the ATS. Workgroup participants were selected on the basis of recognized expertise in the areas of control of breathing, sleep-disordered breathing, sleep, heart failure, PAP, treatment adherence, and population health. Candidate research topics were identified and prioritized on the basis of an iterative process, beginning with a face-to-face meeting of the workgroup in May of 2019, during which currently available evidence for each of the key areas outlined below was reviewed. After discussion, a total of 11 research priorities were identified and are included in this final report. These research priority areas are presented thematically (i.e., not by order of importance).
Findings
Key Area: Control of Breathing and Pathophysiology Leading to CSA
CSA is not a single condition but is rather a manifestation of breathing instability and/or irregularity in a variety of clinical contexts. In contrast to fluctuations in OSA, fluctuations in breathing in CSA are primarily the result of transient changes in central respiratory output, rather than being driven by upper airway obstruction. Research over the past several decades has provided important insights into the neurobiological and physiological mechanisms underlying respiratory control relevant to CSA. This body of work indicates that breathing homeostasis relies on a balance among a number of excitatory and inhibitory influences. However, many unresolved questions remain. Research priorities identified by the workgroup to address gaps in our understanding are discussed below, with examples of specific areas for investigation that may have therapeutic implications provided in Table 1.
Table 1.
Control of Breathing in CSA: Pathogenesis and Potential Therapeutic Targets
| Carotid body | • Chemoreflex role in overall sympathetic activation in heart failure: common pathways |
| • Purinergic signaling | |
| • Gasotransmitters | |
| Brain stem | • Understanding complex integrative responses: RTN, pre-Bötzinger complex, etc. |
| • Mechanisms underlying neuroplasticity: long-term facilitation and potentiation | |
| • Cerebral vasoreactivity: mechanisms and role of impairment | |
| Sleep state and arousals | • Mechanisms of stability in REM |
| • Arousal contribution to instability in CSA | |
| Low cardiac output | • Shear-stress sensing in the carotid body |
| • Interactions between circulatory delay and loop gain; heart–lung interactions | |
| • Fluid shifts in supine position | |
| • Mechanisms sensing pulmonary congestion | |
| System-integrative | • Refined in silico models |
| • CSA model organisms | |
| • Effects of aging, sex, and other systemic influences (e.g., metabolic dysregulation) |
Definition of abbreviations: CSA = central sleep apnea; RTN = retrotrapezoid nucleus.
Research priority 1: advance basic and translational research regarding control of breathing
Control of breathing is complex, involving many sensory and high-order inputs (afferents), central processing areas, and key outputs (efferents); a full discussion is beyond the scope of this manuscript, and readers are referred to contemporary reviews (8, 9). The most widely recognized sensory inputs include the carotid and brain-stem chemoreflex sensors, which detect fluctuations in PaCO2, pH, and PaO2, with varying time scales for responsiveness (Figure 1). Other inputs include those relating to muscle activity (key for exercise), proprioception (e.g., Hering-Breuer reflex, lung stretch receptors), and “higher-order” inputs relating to volitional effort and emotion. Breathing during wakefulness is also substantially different from breathing during sleep, with sleep stages, including those during non-REM and REM sleep, having strong effects on breathing control. Relevant central integration areas are located in the brain stem (largely the medulla) and include the retrotrapezoid nucleus and the pre-Bötzinger complex (9). Key outputs include motor nuclei driving phrenic and hypoglossal nerve activity, together with those innervating the external intercostal, scalene, and sternocleidomastoid muscles. Finally, this system exhibits remarkable and substantial plasticity in sensory components, integrating centers, and output nuclei. Established factors stimulating plasticity include intermittent hypoxemia, inflammation, and aging (10, 11).
Figure 1.
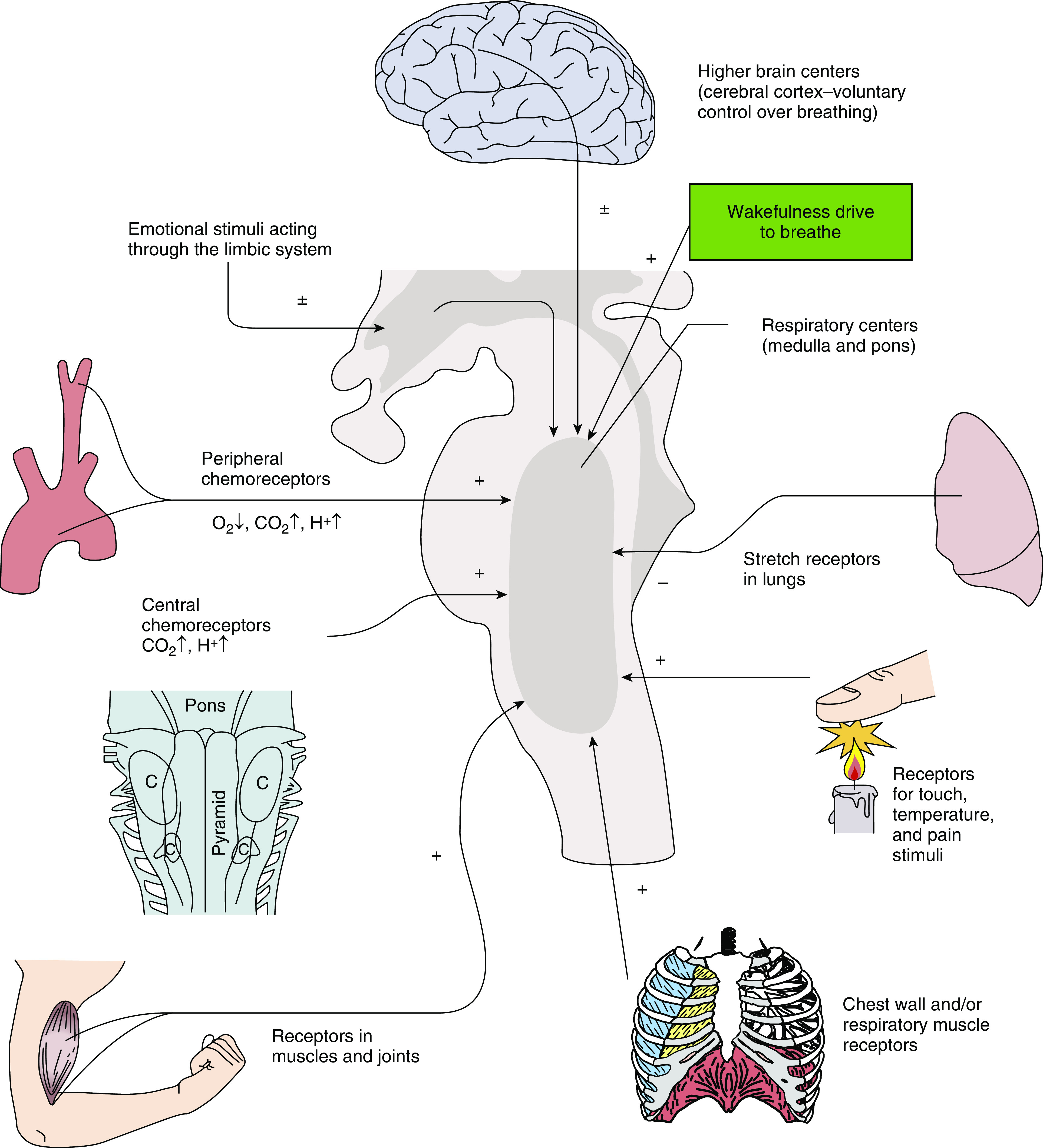
Schematic of ventilatory-control-system inputs/afferents that converge on integrating respiratory centers in the pons and medulla. Chemoreceptors include both peripheral and central centers. Other physical inputs to breathing include lung stretch and irritant receptors; movement/stretch receptors in muscles and joints, including receptors within the chest wall, larynx, and respiratory muscles (including in the upper airway); and peripheral pain receptors. Higher brain centers impact breathing via volitional drive, emotion, and sleep-versus-wake state (wakefulness drive to breathe). + = stimulate; − = inhibit; ± = stimulate or inhibit; C = location of respiratory groups in the medulla. Adapted by permission from Reference 145.
The overall responses of these respiratory-control components determine the persistence of rhythmic breathing or the development of breathing instability and recurrent central apnea. Broadly, CSA may be a consequence of low-ventilatory-drive states (often associated with concurrent arterial hypercapnia) leading to intermittent dropout of ventilatory output or may be a consequence of a hyperresponsive system leading to instability (often associated with concurrent arterial hypocapnia) (12–18). The latter appears to be generally more common and accounts for CSA in the settings of heart failure, CSA observed at high altitude (i.e., environmental hypoxia), idiopathic CSA (i.e., no identifiable underlying cause), emergent CSA in patients with OSA treated with continuous PAP (CPAP), and periodic breathing in infancy.
A concept borrowed from engineering describes the ability of the ventilatory system (brain-stem controller activating the controlled airway, lungs, and chest wall) to maintain stability. A value of gain (also called “loop gain”) is defined by the ventilatory-drive reduction for any prior increase in ventilation, or, similarly, the increase in drive after a decrease in ventilation; a threshold value of above 1.0 represents a level of higher responsiveness that experimentally and computationally can produce periodic central (i.e., nonobstructive apnea [“instability”]) in response to a transient disturbance. Lower values of loop gain may lead to unstable breathing in the presence of ongoing disturbances, including random fluctuations in breathing observed in biological systems (19). Loop gain can become elevated by increased chemoreflex responses to blood gas changes (Po2 and/or Pco2; manifest as a narrow gap between resting CO2 and the “apneic threshold”), increased plant gain (e.g., low lung volume or high Pco2) and/or greater circulatory delay. Although the precise mechanisms contributing to instability may vary by specific etiology, most conditions involve augmented chemosensitivity.
Data supporting the importance of respiratory control responses to the development of CSA include several observations. Experimentally, central apnea in sleeping humans can be induced using mechanical ventilation to decrease PaCO2 below the hypocapnic apneic threshold (20). The difference between the eupneic PaCO2 and the PaCO2 that demarcates the apneic threshold is referred to as the CO2 reserve; a smaller CO2 reserve leads to an increased propensity to develop central apnea. The propensity to develop central apnea varies across physiological and pathological conditions. For example, the CO2 reserve is smaller in men than in premenopausal women, smaller in postmenopausal women than in premenopausal women, smaller in older adults than in young adults, and smaller in patients with OSA than in individuals without OSA (21–23). These differences seem to contribute to a variable prevalence of CSA in each population. In addition, there is marked plasticity in the propensity to develop central apnea. For example, administration of testosterone to premenopausal women results in a decreased CO2 reserve (24). Conversely, administration of synthetic gonadotropin-releasing hormone results in an increased CO2 reserve in men (25). Other examples of plasticity include changes in the CO2 reserve in response to treatment with finasteride, PAP therapy in patients with OSA, acute intermittent hypoxia, and acute hyperoxia (22, 26–28). Nonetheless, a disconnect remains between these physiological studies and an understanding of the associated neurobiological pathways.
The importance of the wake-versus-sleep state in control of breathing is also established. Specifically, non-REM sleep removes the wakefulness “drive to breathe” and renders respiration critically dependent on chemical influences, especially PaCO2. In addition, there is unmasking of a threshold Pco2, a level below which ventilation ceases. Thus, a small drop in prevailing Pco2 causes central apnea during sleep. Clinical observations include the destabilizing effect of wake–sleep transitions and dramatic changes in respiratory-event frequencies in non-REM versus REM sleep. Again, little is known regarding the pathways that connect wakefulness and cortical activity, subcortical sleep-promoting areas, or brain-stem control of breathing.
Research priority 2: determine mechanisms that mediate breathing instability in CSA
Despite substantial progress in understanding both the control of breathing and in the physiology of CSA, neurobiological mechanisms by which heart failure leads to CSA remain poorly understood.
The most consistent pathophysiological determinant of CSA in heart failure is increased chemosensitivity to CO2 and hypoxia (12–18), typically measured during wakefulness. CSA severity is more strongly associated with dynamic CO2 responses (designed to measure carotid body/peripheral chemosensitivity) than with responses measured from the slower rebreathing method (designed to assess predominantly central CO2 responses) (13). Consistent with this view, carotid body denervation in rabbits with cardiac ventricular pacing–induced heart failure has also been shown to resolve CSA, suggesting that heightened peripheral chemosensitivity is required for CSA to occur. Interestingly, CSA in patients with heart failure is equally well correlated with ventilatory responses to CO2 and hypoxia; when both responses are augmented, CSA becomes particularly severe (18).
The precise pathway between heart failure and augmented chemosensitivity in CSA remains unclear. Chemoreflex sensitivity has been related to increased left heart filling pressures and pulmonary congestion in humans (29, 30). Of note, a lower DlCO may be a marker of such congestion (31). In addition, changes in the pulmonary blood volume from wakefulness/upright positioning to sleep/supine positioning may relate to fluid movement from the periphery toward the lung (“rostral fluid shift”) (32). Alternatively, in a rabbit model, low carotid blood flow independent of cardiomyopathy leads to diminished shear stress in the carotid body, augmented chemosensitivity, and emergent CSA (33). Biological determinants of excessive hyperreflexia at the carotid bodies (e.g., purinergic signaling) in heart failure are an important therapeutic target in need of focused, basic, scientific research.
Other factors beyond blood gas chemoreflex sensitivity per se can raise the functional ventilatory responsiveness to blood gases (and thus loop gain) and promote CSA, but the relative importance remains unclear in CSA in those with heart failure (12).
-
1.
In patients with heart failure, low cardiac output contributes to a substantial delay between changes in PaCO2 (and also PaO2) in the pulmonary capillaries and respiratory centers in the carotid body (and brain stem), which increases loop gain (toward the critical threshold of 1.0) and provides the background preconditions for CSA (34–37). Indeed, patients with heart failure and a lower left ventricular ejection fraction (LVEF)/cardiac output, atrial fibrillation, and greater circulatory delays are typically at heightened risk of CSA (1, 5, 33, 38). Reducing circulatory delay with heart failure therapies can also improve or ameliorate ventilatory oscillations (39–41), yet it is unclear the extent to which circulatory delay itself (rather than changes in peripheral and/or central chemosensitivity) contributes to CSA.
-
2.
Impaired cerebral vasoreactivity to CO2 can (in principle) hinder the damping of CO2 swings in the brain stem, yielding augmented responses to swings in arterial Pco2.
-
3.
Reaching the threshold to arousal from sleep acts to raise the functional responsiveness and loop gain (21, 42–46). Of note, CSA is most common in stage 1 non-REM sleep, in which sleep is fragile and arousals are frequent. In some patients, however, CSA occurs throughout stages 1–3; recognizing a phenotype of CSA that is arousal dependent may provide an avenue for precision-medicine intervention.
-
4.
A propensity for the upper airway to collapse may also destabilize breathing (47). Patients with a moderately collapsible upper airway are dependent on ventilatory motor output to preserve upper airway patency (48, 49). Accordingly, pharyngeal narrowing or obstruction develops when the ventilatory motor output reaches a nadir (48, 50–54). Mucosal adhesion may impede upper airway reopening, exacerbating the loss of ventilation during “central hypopnea” and further destabilizing breathing (52). Upper airway collapsibility is prevalent in the general population and may be more common in patients with heart failure as a result of pharyngeal fluid accumulation (55), although more mechanistic data are needed. Moreover, there is substantial overlap between CSA and OSA in heart failure, including the presence of both obstructive and central events within many individuals (56).
Key Area: Variability in CSA across Individuals and over Time
Research priority 3: examine CSA in diverse groups and across the adult life span
Increasing age is a well-recognized risk factor for sleep-disordered breathing, most appreciated in OSA but also observed in CSA (57, 58). The most well-established effect of age relates to anatomic changes of the upper airway that might lead to OSA (59). In addition, increasing age is a strong risk factor for the development of heart failure and thus may drive increases in the population prevalence of CSA. However, within those with heart failure, CSA is more common with increasing age, suggesting other effects of aging that might directly contribute to CSA (3). Potential mechanisms require further study, but effects on control of breathing, lung function, and respiratory muscle activity are all potential contributors (60). In terms of sex, epidemiological studies have demonstrated a significantly higher prevalence of CSA in men than in women (61). Physiological studies suggest key differences in control of breathing, with evidence implicating testosterone more so than progesterone. Observations include administration of testosterone to women increasing the propensity to develop central apnea (23–25). Accordingly, the effect of menopause and aging in women may be a key topic (62), particularly as the prevalence of heart disease has greatly increased in women.
The prevalence, pathogenesis, and consequences of CSA are likely to differ across different groups of individuals with heart failure, and optimal management is thus likely to vary. Indeed, the prevalence of CSA (and OSA) among patients with heart failure has been variable across several studies, likely reflecting differences in heart failure severity and patient characteristics (e.g., age, sex, and body mass index). Established risk factors and associations include male sex, increasing age, lower LVEF, and comorbid atrial fibrillation (3). In contrast to large studies of OSA among the general population enrolling a wide spectrum of patients, CSA studies have been relatively small. Key questions remain regarding potential risk factors, such as race and/or ethnicity, medications (not only heart failure drug classes but also concurrent opioids, sedatives, etc.), heart failure etiology, etc.
From the standpoint of associated symptoms and clinical presentation, patients with heart failure and CSA (or OSA) may not report symptoms such as sleepiness or sleep disruption (63–65). Presenting complaints may be attributed to heart failure per se. Bed partners may note apneas or hyperpneas, and patients may report fatigue, insomnia, paroxysmal nocturnal dyspnea, and/or nocturnal angina, although the specificity regarding CSA is unknown. A priority is establishing the spectrum of symptoms using validated questionnaires to assess sleep across domains of sleep disruption and sleep-related impairment (i.e., beyond sleepiness), associated sleep comorbidities such as insomnia and restless leg syndrome, and quality of life. The extent to which these profiles of signs and symptoms differ across individuals by age, sex, heart failure characteristics, and race/ethnicity has not been adequately examined. One specific need that may capitalize on this demographic and clinical data is the development of a robust risk score that can be used to identify individuals with CSA.
Research priority 4: evaluate changes in CSA over time and the relationship with heart failure status
Temporal variability in CSA has not been adequately characterized. CSA appears to be more common in decompensated heart failure, (66) likely based on physiological changes that contribute to breathing instability (e.g., increased left atrial pressure, pulmonary congestion, increased circulation time) (33, 67). However, treatment of decompensated heart failure does not reliably resolve CSA (68). Identifying individuals with persistent CSA may help to define a group that needs sustained CSA therapy versus supportive care or time-limited CSA treatment (see adaptive servoventilation [ASV] treatment below).
Conversely, variability in heart failure over time may be impacted by changes in CSA. Thus, there is clear potential for interactions between these two conditions over time. For example, some have hypothesized that subclinical changes in cardiac function may trigger or worsen CSA, which may lead to further heart failure decompensation. In theory, recognition of and intervention for CSA might present an opportunity to break a downward spiral toward important outcomes, including symptoms and hospitalization. Further research is needed in this area but will require technologies capable of monitoring changes in sleep apnea over time, such as “wearables.”
Key Area: Techniques to Examine CSA Pathogenesis and Outcomes
Research priority 5: develop and validate tools to better characterize CSA (i.e., beyond the AHI)
According to the AASM/International Classification of Sleep Disorders, CSA is defined as at least 5 central events/h that comprise at least 50% of the total AHI (69). For CSA with Cheyne-Stokes respiration, there must be at least three consecutive central events in a crescendo–decrescendo pattern and a cycle length of at least 40 seconds. (Note that the ERS Task Force on CSA prefers the term “periodic breathing in heart failure” instead of “Cheyne-Stokes respiration” to describe the polysomnographic pattern and the underlying disease more precisely and to avoid unclear historical terms.) Thus, the criteria rest largely on the AHI and the presence of a typical pattern of breathing. Nonetheless, there may be considerable variability between individuals in features of breathing, which may have important implications. Indeed, recent investigations into various traits have shed new light on OSA pathophysiology and treatment (i.e., arousal threshold, upper airway responsiveness) (70–72). These concepts have not yet been applied to CSA but are likely to be insightful (56). As the current tools to estimate key traits responsible for sleep-disordered breathing have focused on OSA, including recent signal extraction of endophenotypes from polysomnography signals, the development and validation of CSA-specific surrogates to predict outcomes and inform treatment approaches is a priority for future CSA research.
There are two major reasons to identify and quantify aspects of CSA pathophysiology beyond a count of the frequency: 1) to characterize the pathogenesis for the purposes of understanding how to treat the disorder and 2) to better determine the risks associated with the untreated disorder.
Cycle duration
In heart failure, cycle durations are typically 40–90 seconds, with longer cycle lengths seen in patients with lower LVEF, lower cardiac output, and greater circulatory delays (73). Treatments may be dependent on cycle duration; for example, central events with longer cycle lengths have slower fluctuations in blood gases and thus may garner input from central chemoreflexes such that supplemental oxygen could be less effective. Conversely, cycle duration may provide important information about the presence versus absence of heart failure (34).
Central versus obstructive contributions
The extent to which sleep apnea is driven by central versus obstructive components is challenging to ascertain and has therapeutic implications. CPAP is likely to fail in those with a very high loop gain (74). Therapies for the central contribution, such as diaphragm pacing, oxygen, acetazolamide, or new drugs to lower carotid chemosensitivity, are likely to fail in those with a substantial obstructive component. Although some events are clearly obstructive (clear thoracic and abdominal excursions during apnea) and others clearly central (no excursions), many events have a combined etiology. Recently, we have seen the beginning of new methods to estimate the central versus obstructive nature of events (e.g., based on flow limitation, model-estimated chemical drive, or periodic/sinusoidal nature of oxygen saturation as measured by pulse oximetry) (75). To limit the challenges associated with further research in this area, we implore researchers to use the highest-quality unfiltered signals (e.g., direct coupled nasal pressure, true direct coupled respiratory inductance plethysmography; signals must “hold a baseline”). Validation of any new definition or method should involve prediction of responses to therapies for CSA.
Loop gain and ventilatory pattern
The higher the loop gain underlying CSA, the longer the duration of apnea relative to the total cycle duration. In small physiology studies, this “duty-ratio” measure of loop gain has been found to be higher in patients with heart failure who were nonresponsive to CPAP and respiratory stimulation (74). Alternative methods for predicting high loop gain have been proposed, including examining awake breathing, examining sighs, and quantifying the presence or absence of stable breathing (76, 77). Tailoring the magnitude of an intervention to the severity of the underlying magnitude of control instability thus has promise. Finally, changes in end-expiratory lung volumes (EELVs) during the hyperpnea phase of CSA may also be relevant, although detection requires unfiltered true respiratory inductance plethysmography, which is not standardized.
Sleep state instability dependence
Some patients exhibit CSA nearly exclusively during stage 1 sleep and exhibit profound dropout of ventilation in time with sleep onset, with ventilation resuming upon arousal. Recognizing these individuals on the basis of the synchrony between EEG measures of wakefulness and ventilation may help identify patients who might benefit from interventions that promote sleep (hypnotics). However, the relationship between abnormal respiratory events and arousal may be bidirectional. In other words, failure to reach non-REM stage 2 or 3 may be due to recurrent apnea/hypopnea. Moreover, the potential for an adverse effect of hypnotics on ventilation in patients with abnormal respiratory status, including patients with morbid obesity, has to be carefully examined.
Oxygenation and arousal impacts
Central apnea results in cycles of hypoxia and reoxygenation and transient arousals from sleep. Oxyhemoglobin desaturation in the aftermath of apnea/hypopnea can be objectively captured and analyzed. Several potentially relevant parameters can be extracted from the oximetry signal, including the number of oxygenation dips, the mean and nadir saturation, and the percentage of total sleep time with a saturation < 90%. Other innovative metrics include the sleep apnea–specific hypoxia burden, which takes into consideration the area under the curve of the oxygen desaturation temporally related to respiratory events, and the “hypoxemic burden,” which has been associated with outcomes in large studies of patients with OSA (78). Sleep state disturbance, as measured by the frequency of EEG arousals, has been associated with apnea-related sympathoexcitation. Prolonged circulation time may also have important prognostic implications (73).
Research priority 6: determine how sleep apnea might contribute to, or protect against, heart failure progression
CSA is independently associated with poor outcomes in patients with heart failure; the severity of CSA may be a better indicator of mortality than clinical characteristics, echocardiographic characteristics, or other patient characteristics (79). Although the possibility exists that CSA is merely a marker of heart failure severity, several lines of evidence suggest that CSA per se contributes to adverse outcomes. Major clinical outcomes are discussed more below but include the outcome that CSA treatment with PAP therapy improves the LVEF and nocturnal ventricular arrhythmias in patients in whom CPAP suppresses CSA (80, 81).
Autonomic activation is a key feature of heart failure as well as sleep-disordered breathing (in both CSA and OSA). Evidence that this pathway may mediate the negative effects of CSA include the observation that CSA treatment improves catecholamine concentrations (82, 83) and ventricular arrhythmias (80). Conversely, intermittent hypoxemia has important effects, including promoting diurnal hypertension. Intrathoracic pressure swings mediate arousal but also increase transmural cardiac stress. Swings in CSA are generally smaller than those seen in OSA, but the spectrum observed across individuals may be useful for investigating the significance of intrathoracic pressure changes.
Another hypothesis that has been put forth is that CSA might be an adaptive response in heart failure, at least in some individuals (84). The proposed physiological advantages of CSA included increased lung volume, avoidance of hypercapnic acidosis, bronchodilation, and cyclical ventilatory muscle rest. Although evidence remains sparse, recent investigations have found evidence to support this concept. Perger and colleagues examined EELV in patients with heart failure and CSA and found two patterns during hyperpnea—a positive pattern (i.e., preserved or increased EELV during hyperpnea) and a negative pattern (i.e., reduced EELV due to presumed expiratory muscle recruitment) (85). Patients with the negative pattern tended to have worse cardiac function. It was proposed that the reduced lung volume and increased intrathoracic pressure could represent a cardiac “autoresuscitation” mechanism that increases stroke volume in response to reduced preload and decreased left ventricular transmural pressure. This group subsequently provided evidence that stroke volume falls less in patients with the negative pattern than in patients with the positive pattern (86). Although these data are preliminary, the findings support the concept that there may be subgroups of patients with heart failure in whom CSA has adaptive benefit. The results of SERVE-HF (ASV for CSA in Systolic Heart Failure) (discussed more below) that demonstrated harm associated with treatment of CSA, particularly among certain groups, emphasize the importance of determining in which contexts CSA should be treated versus not treated.
The potential “downstream” mechanistic cardiac consequences of CSA physiology/pathophysiology require exploration, including via imaging studies (e.g., magnetic resonance imaging studies of myocardial fibrosis), novel heart failure biomarkers (e.g., ST2), and multiomics approaches. The extent to which negative or positive cardiovascular changes are driven by intermittent hypoxemia, arousal, lung volumes, respiratory muscle patterns, or intrathoracic pressure changes can be examined more precisely using endophenotyping techniques noted above or by alternatively using model organisms to isolate these factors.
Key Area: Impact of Device and Pharmacological Treatment
A summary of therapeutic options and relevant clinical questions is provided in Table 2.
Table 2.
Current Questions about Potential Treatments for CSA
| Potential Treatments for CSA | Current Question(s) |
|---|---|
| Heart failure therapies | • Is CSA a mediator or modulator of benefit? |
| CPAP | • Is CPAP effective in predicted responders, and who responds? |
| • Should CPAP be used as a standard comparator? | |
| Adaptive servoventilation | • Are there identifiable device and/or patient-level factors predicting harm or benefit? |
| • Does better efficacy improve adherence? | |
| Inspired CO2 | • What is the clinical feasibility? |
| • Are there adverse effects related to hypercapnia or increased ventilation? | |
| Supplemental oxygen | • Who will respond (AHI, symptoms, end-organ, etc.) to supplemental O2? |
| • Are there issues with adherence, and what strategies improve adherence? | |
| Phrenic pacing | • What are the long-term outcomes and comparative effectiveness (including the “effective AHI”)? |
| Pharmacotherapy | • What are the most promising targets? |
| • Is there a role for combination or “rescue” therapy? |
Definition of abbreviations: AHI = apnea–hypopnea index; CPAP = continuous positive airway pressure; CSA = central sleep apnea.
Research priority 7: determine the impact of established and emerging heart failure therapies on CSA
Effective heart failure treatment can clearly improve and even resolve CSA by reducing chemoreflex sensitivity, improving lung volume, and decreasing circulatory delay. Relatively small-scale (and generally investigator-initiated) studies have shown that medical management, cardiac resynchronization, left ventricular assist devices, and cardiac transplantation improve (but do not always resolve) sleep apnea (39, 87, 88). Novel therapeutic strategies that may impact CSA should be considered for rigorous investigation:
-
1.
Carotid body denervation has been recently gained renewed interest as a potential therapy for heart failure (89). Disruption of chemoreflex responses may have advantageous effects via decreasing sympathetic tone, but the importance of carotid bodies in breathing clearly mandates that CSA be considered in this therapeutic approach, both as a potential mediator of any beneficial effects and as a potential adverse consequence. Potential unintended adverse consequences of carotid body denervation may not manifest for many years. Indeed, as weight gain and comorbidities increase with age, so does the presence and severity of sleep-disordered breathing, which may place these individuals at increased risk of respiratory failure during sleep, in which chemical control of breathing dominates.
-
2.
Cardiac rehabilitation is another intervention that can have a substantial impact on quality of life in those with heart failure. Physical activity and diet may have an impact on sleep apnea via several pathways, and improvements in CSA might thus account for at least some of the benefits of rehabilitation (90). The potential for concurrent sleep apnea treatment with rehabilitation is another promising area, given the potential interactive effects.
It remains unclear whether CSA represents an independent outcome of heart failure and, if so, whether titrating heart failure therapy to achieve resolution of CSA may be a goal. Nonetheless, the group felt strongly that inclusion of CSA as a key outcome in heart failure therapy studies is strongly warranted, including within industry-sponsored pharmaceutical and device studies. Such data will help to determine whether resolution of CSA is associated with improved outcomes and, similarly, whether the impact of the examined heart failure therapy is modulated by the presence or absence of CSA.
Research priority 8: clarify the role of PAP therapy for CSA in those with heart failure
CPAP is considered an established therapy for OSA, but it also has physiological effects that may improve CSA by increasing lung volumes, decreasing preload and afterload, and stabilizing the upper airway. In addition, concomitant upper airway collapse and OSA is common in many patients with CSA; accordingly, treatment with CPAP may improve CSA and the overall disease severity. Nonetheless, the CANPAP (Canadian CPAP for Patients with CSA and Heart Failure) study found no overall improvement in survival with use of CPAP in patients with CSA (91). However, in post hoc analysis, those with a reduction in the AHI to less than 15 events/h had improved survival with treatment (81). Current AASM and ERS guidelines recommend CPAP use for patients with CSA due to heart failure, provided CPAP use results in a similar reduction in the AHI. Because these recommendations are based on post hoc analysis, a prospective study of CPAP in those with heart failure that enrolls individuals likely to respond to CPAP is needed to make a firm recommendation. Such a study will be aided by aforementioned physiological predictors of CPAP responsiveness.
On the basis of the suboptimal response to CPAP in terms of AHI reduction among a substantial number of individuals, noninvasive ventilation has been explored as a therapeutic option. ASV is a modality specifically designed to control CSA by augmenting ventilation during hypopnea or apnea, which leads to an overall stabilizing effect on breathing. In those with CSA due to heart failure, ASV is highly efficacious at reducing the AHI, but clinical outcomes have been mixed. Results of a large trial, the SERVE-HF study, found that among patients with heart failure and a reduced LVEF (LVEF < 45%, termed HFrEF), ASV led to increased overall mortality (92). Therefore, ASV is now contraindicated for clinical use in patients with HFrEF, noting that this was the select group of individuals with moderate-to-severe CSA (i.e., AHI > 15 events/h, >50% of events being central in nature, and at least 10 central apneas/h). A contemporaneous study examining the response to ASV in acutely decompensated heart failure (CAT-HF [Cardiovascular Outcomes with e-targeted ASV Therapy in Heart Failure]) was stopped early on the basis of SERVE-HF results. Although the CAT-HF results were negative in terms of the primary composite outcome, the subgroup of patients with preserved LVEF (termed “heart failure with preserved ejection fraction”) appeared to have a benefit (93). The ongoing ADVENT-HF (Effects of ASV on Survival and Frequency of Hospital Admissions in Patients with Heart Failure and Sleep Apnea) trial will further examine the use of ASV in HFrEF, with potentially important trial-design differences including differences in the enrollment of patients with OSA, the ASV settings (i.e., lower default pressures), and the manufacturer algorithms (94). Importantly, the algorithms used by the ASV device to stabilize breathing (e.g., speed at which pressure support is increased) may be relevant to therapy responses (95).
Despite substantial investigation of the SERVE-HF data, risk factors and associated mechanisms by which ASV might lead to harm remain unclear (96, 97). Additional mechanistic data regarding effects of ASV on cardiac preload and/or afterload, neurohormonal changes, biomarkers, etc., may help with better understanding device effects and with defining individuals who might benefit versus those who might be harmed (98, 99).
Neural stimulation, employing unilateral phrenic-nerve stimulation, has been approved by the Food and Drug Administration for treatment of CSA, including in patients with heart failure. A randomized controlled trial showed improvement in the AHI by approximately 50%, near elimination of central apneas, and some subjective improvements in symptoms, together with other potential beneficial effects (100, 101). Nonetheless, long-term outcomes, including the effect on key heart failure outcomes and mortality, have not been examined. In addition, response rates remain suboptimal, likely because of the fact that upper airway collapse is not addressed with the device. AHI thresholds for determining successful treatment are not well established, and predictors of response versus nonresponse need to be established, potentially including the above-mentioned phenotyping techniques.
Research priority 9: establish the utility of supplemental oxygen, inspired CO2, and pharmacotherapy for treatment of CSA
The challenges associated with PAP device use (including effectiveness and adherence issues) in patients with CSA has highlighted the critical need for alternative treatments for CSA. The major targets for pharmacotherapy of CSA in general are addressing elevated chemoreflex sensitivity, elevated plant gain, and arousal responses (56).
Beyond CPAP, supplemental oxygen is often considered for CSA in patients with heart failure on the basis of AASM guidelines, although long-term data are lacking. The LOFT-HF (Impact of Low-Flow Nocturnal Oxygen Therapy on Hospital Admissions and Mortality in Patients with Heart Failure and CSA) trial recently started enrollment, which is an NIH phase 3 trial in which patients with HFrEF with predominant CSA are randomized to receive either oxygen from a concentrator or a sham (airflow from a concentrator) using a titration protocol designed to limit potential oxygen toxicity. Endpoints include hospitalization due to heart failure and mortality. Even with a positive response, prior physiological data suggest that supplemental oxygen will not suppress CSA in all individuals, and therapy thus may need to be individualized.
Delivery of CO2 was proposed several decades ago as a strategy to stabilize breathing in CSA (102, 103). An increase in inspired CO2 can be achieved either with exogenous CO2 (i.e., from a tank) or via rebreathing of exhaled CO2. Both approaches can stabilize breathing via a decrease in the efficiency of CO2 excretion (i.e., lower “plant gain”). Limitations of real-world use of exogenous CO2 include the need for a continuous supply of tanks and potentially include the development of adverse effects related to hypercapnia, which may be mitigated by systems that only deliver CO2 during the hyperpnea phase (104). Other potential adverse effects include an increased drive to breathe with attendant increases in intrathoracic pressure swings. Similarly, rebreathing CO2 via increases in applied dead space requires careful titration, which may be facilitated by titratable devices (105). In one application, dead space added to a CPAP mask for those with persistent central events was able to improve CSA (106). Endotyping techniques, as noted above, in a proof-of-concept study have been shown to predict the response to inhaled CO2 (107). Further advances are needed in terms of patient selection, technologies for practical application, and studies examining the impact of this strategy on patient-oriented outcomes.
Given the primary role of control of breathing in CSA, pharmacotherapies that impact control of breathing are a promising approach. A number of medications have been proposed, such as acetazolamide, zolpidem, buspirone, and others (108). The respiratory stimulant acetazolamide has been best studied and has been used in other CSA conditions such as CSA at altitude (109, 110). Several short-term studies have explored the use of acetazolamide as a treatment for CSA (111–114). Physiologically, acetazolamide results in decreased plant gain by increasing alveolar ventilation and thereby lowering Pco2, but acetazolamide has a limited effect on CO2 chemoreflex sensitivity (115, 116). Zolpidem, a nonbenzodiazepine hypnotic has been used clinically for the treatment of CSA based on one open-label study in patients with idiopathic CSA (117). Buspirone acutely increases ventilation during wakefulness and non-REM sleep in rodent models (118, 119). There are a few clinical reports of its benefit (120–122). Although these agents show promise in the treatment of CSA in general, their safety and efficacy in heart failure are still unknown, and additional research in this subset of patients with CSA is needed. Furthermore, effects from using many of these agents as monotherapies have been modest, suggesting a need for new drugs or combination strategies.
Studies are also needed to explore other novel candidate pharmacological targets: 1) KLF2, signaling shear stress in the carotid (123); 2) inflammation-related signaling (e.g., via TNFα and IL-6) in both the carotid body and central nervous system (10, 124, 125); 3) carotid gasotransmitters CO and H2S (126); and 4) P2X3 receptors, modulating autonomic receptor sensitivity (127). Notably, given that the carotid chemoreflex also protects against hypoxia, an intervention is needed that reduces carotid body hyperreflexia but preserves its physiological function. In contrast, removing the carotid function completely with bilateral carotid denervation leads to worsening oxygenation (128). It is feasible that aforementioned pharmacological targets may achieve this goal, but other undiscovered mechanisms may be involved. As discussed above, further investigation is needed to elucidate underlying mechanisms to inform development of novel therapeutics for CSA.
Key Area: Implementing CSA Treatments for All Individuals
Research priority 10: determine factors influencing adherence to CSA treatments
Adherence to long-term therapy, including adherence to medications for chronic diseases such as hypertension, is suboptimal (129). Several studies have shown that long-term daily use is achieved by only 50–80% of patients (130). Evidence on treatment adherence in patients with CSA is limited, in contrast to substantial investigation in patients with OSA (131). Importantly, CSA is not fully controlled with CPAP in many individuals, which would be expected to lead to reduced adherence. Indeed, some data suggest that treatment efficacy may impact adherence, including a recent analysis in which switching from CPAP to ASV showed increased adherence over a 60-day window after treatment was changed (132).
With regard to ASV, in the SERVE-HF study, adherence (defined as 3 h or more per night) was 60%, with mean nightly use of 3.7 hours, and 27% of patients did not use treatment at all (defined as <1 h per night). This rate of adherence is similar to what has been seen in trials in OSA (133), although usage seems to be substantially higher in the ongoing ADVENT-HF trial based on interim analysis (134). Uncertainty remains regarding how adherence might be impacted by intrinsic differences between ASV and CPAP (e.g., additional settings that must be optimized), differences across ASV devices, or differences based on the efficacy in the treatment of CSA.
Studies investigating adherence to non-PAP therapy are limited. In a systematic review of oxygen therapy, four studies that included use of oxygen therapy for several months found acceptable adherence, perhaps because the nasal cannula was more comfortable than a face mask, as would be required for PAP therapies (135). In a large, randomized trial among patients with OSA, there was a mean duration of use of supplemental oxygen (4.8 h per night) that was higher than that of CPAP (3.5 h per night) (136); however, it is not clear whether objective data were available for hours of use of oxygen as they are for CPAP treatment. Overall, measures to objectively quantify oxygen use and enhance adherence to supplemental oxygen are needed.
Additional research on adherence to therapy specifically in CSA is warranted, first to understand barriers to daily use and then to understand whether symptom relief is a predictor of increased use in these patients. Furthermore, although there is some evidence for improved adherence to ASV compared with CPAP, there are no blinded or crossover studies to confirm the findings from cross-sectional research. Studies are also needed to translate to CSA the known effective strategies for increasing PAP use among patients with OSA, such as educational, supportive, and behavioral interventions (137). Identifying specific challenges to adherence with non-PAP therapies such as oxygen and pharmacotherapy will clearly be needed to effectively implement such treatments. Lastly, not all treatments are equally dependent on the patient actively engaging with the device; for example, phrenic-nerve stimulation is relatively “automatic” in that once turned on for the night, no further engagement is needed. Accordingly, research should make clear distinctions between the efficacy (i.e., control of AHI during use) and effectiveness (i.e., considering use pattern or the “effective AHI”) when comparing treatment strategies.
Research priority 11: characterize health disparities related to CSA across populations
A health disparity is defined as “a health difference that adversely affects defined disadvantaged populations, based on one or more health outcomes” (138). In this context, health outcomes are characterized as a higher incidence or prevalence of disease, including earlier onset or more aggressive progression; premature or excessive mortality from specific conditions; greater global burden of disease, such as disability-adjusted life years, as measured by population health metrics; poorer health behaviors and clinical outcomes related to the aforementioned information; or worse outcomes on validated self-reported measures that reflect daily functioning or symptoms from specific conditions. Of note, minority health is defined as “health characteristics and attributes of racial and/or ethnic groups who are socially disadvantaged due in part to being subject to potential discriminatory acts” (139). Analogous to disadvantaged populations, the following are NIH-designated U.S. health-disparity populations based on the degree of social disadvantage due to historical and contemporary discriminatory policies and practices: Black individuals/African Americans, Hispanic/Latino individuals, American Indians/Alaska Natives, Asian Americans, Native Hawaiians and other Pacific Islanders, socioeconomically disadvantaged populations, underserved rural populations, and sexual and gender minority groups.
A recent workshop report highlighted research gaps, challenges, and opportunities in sleep and health-disparity research that are important to advance the emerging field of sleep-health disparities among designated U.S. health-disparity populations (140). Given the complex and multifactorial determinants of health disparities, future sleep-disparity research should focus on health-disparity causal pathways, along with sleep and circadian rhythm–related mechanisms, to better understand sleep-health disparities. Strategies identified from the workshop included 1) focusing on sociocultural and physical/built environmental determinants of sleep health to understand the underlying contextual factors to target disparities in sleep, 2) better integration of sleep and health-disparity research field theories and methodologies, and 3) designing multilevel interventions through transdisciplinary teams focused on addressing sleep-health disparities.
Data related to CSA are sparse among all populations disproportionately impacted by disparities. Focusing on racial and ethnic minorities as an illustrative example, these groups (especially Black individuals/African Americans) disproportionately experience sleep-disordered breathing (e.g., OSA, asthma), heart failure, and comorbidities (e.g., hypertension, which increases stroke risk) that could impact CSA (141–143). For instance, Bahrami and colleagues found that the higher incidence of congestive heart failure among Black individuals/African Americans was related to a higher prevalence of hypertension, diabetes, and lower socioeconomic status (144). The authors also found that heart failure biological mechanisms differed by race/ethnicity in that an increase in left-ventricular mass had the greatest impact in white individuals and Hispanic/Latino individuals and in that myocardial infarction was the least influential among Black individuals/African Americans. Reasons for differences in underlying mechanisms should be identified, as determinants may differ and differences in treatment may be necessary. Other aspects deserving consideration for treatment of sleep apnea are the cost of therapy and health-literacy requirements. Disparities in CSA specifically are likely to exist, but more research is needed.
The following are recommendations for future research to be conducted to answer the aforementioned questions:
-
•
Include sufficient sample sizes of populations disproportionately impacted by health disparities in future research studies on the topic and conduct of race-specific investigations.
-
•
Collect data on “upstream” (close to the root cause) factors like social (e.g., socioeconomic factors, healthcare access) and environmental (e.g., air quality affecting pulmonary comorbidities) determinants of health and health disparities to investigate their impact on CSA risk, diagnosis, and treatment in the overall population and in terms of their contributions to health disparities.
Even if disparities in CSA are not observed, it will remain important to explicitly investigate and intervene in health-disparity populations, as the general health of these populations tends to be worse and there is a need to concentrate efforts on improving minority health. The ultimate goal is achieving sleep-health equity, which has been defined as “equal opportunities that are given to each individual and/or communities based on their need, no matter their age, sex, race/ethnicity, geographic location, and socioeconomic status, to obtain recommended, satisfactory, efficient amount of sleep with appropriate timing that promotes physical and mental well-being” (139).
Conclusions
Advancing care for patients with CSA in the setting of heart failure will require progress in the arenas of translational (basic through clinical), epidemiological, and implementation research. Given the increasing prevalence of heart failure and its associated substantial burden to individuals and society as well as the healthcare system, targeted research strategies to improve knowledge of CSA pathogenesis and treatment are a priority.
Supplementary Material
Acknowledgments
This research statement was prepared by an ad hoc subcommittee of the ATS Assembly on Sleep and Respiratory Neurobiology.
Members of the subcommittee are as follows:
Jeremy E. Orr, M.D. (Co-Chair)1
M. Safwan Badr, M.D. (Co-Chair)2,3
Indu Ayappa, Ph.D.4
Danny J. Eckert, Ph.D.5
Jack L. Feldman, Ph.D.6
Chandra L. Jackson, Ph.D., M.S.7,8
Shahrokh Javaheri, M.D.9,10
Rami N. Khayat, M.D.11,12,13
Jennifer L. Martin, Ph.D.14,15
Reena Mehra, M.D.16,17,18,19
Matthew T. Naughton, M.D.20
Winfried J. Randerath, M.D.21
Scott A. Sands, Ph.D.22
Virend K. Somers, M.D., Ph.D.23
1Division of Pulmonary, Critical Care, and Sleep Medicine, University of California, San Diego, San Diego, California; 2John D. Dingell Veterans Affairs Medical Center, Detroit, Michigan; 3Department of Internal Medicine, Wayne State University, Detroit, Michigan; 4Division of Pulmonary, Critical Care and Sleep Medicine, Icahn School of Medicine at Mount Sinai, New York, New York; 5Adelaide Institute for Sleep Health, Flinders Health and Medical Research Institute, College of Medicine and Public Health, Flinders University, Adelaide, South Australia, Australia; 6Department of Neurobiology, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, California; 7National Institute of Environmental Health Sciences, NIH, Durham, North Carolina; 8Intramural Program, National Institute on Minority Health and Health Disparities, NIH, Bethesda, Maryland; 9Division of Pulmonary and Sleep Medicine, Bethesda North Hospital, Cincinnati, Ohio; 10Division of Pulmonary, Critical Care and Sleep Medicine, University of Cincinnati, Cincinnati, Ohio; 11University of California, Irvine, Sleep Disorders Center and 12Division of Pulmonary and Critical Care, University of California, Irvine, Irvine, California; 13The Sleep Heart Program, Ohio State University, Columbus, Ohio; 14Veterans Affairs Greater Los Angeles Healthcare System, Los Angeles, California; 15David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, California; 16Sleep Disorders Center, Neurological Institute, 17Respiratory Institute, 18Heart and Vascular Institute, and 19Lerner Research Institute, Cleveland Clinic, Cleveland, Ohio; 20Department of Respiratory Medicine, Alfred Hospital–Monash University, Melbourne, Victoria, Australia; 21Institute of Pneumology, University of Cologne–Bethanien Hospital, Solingen, Germany; 22Division of Sleep and Circadian Disorders, Brigham and Women’s Hospital–Harvard Medical School, Harvard University, Boston, Massachusetts; and 23Department of Cardiovascular Medicine, Mayo Clinic, Rochester, Minnesota
Footnotes
Supported by the American Thoracic Society; the Intramural Program at the NIH, National Institute of Environmental Health Sciences (Z1A ES103325-01); NIH grant R01HL130552; and Veterans Health Administration Office of Research and Development grant I01CX001040.
This official research statement of the American Thoracic Society was approved December 2020
An Executive Summary of this document is available at http://www.atsjournals.org/doi/suppl/10.1164/rccm.202101-0190ST.
Author Disclosures: J.E.O. received a partnered grant from the ATS Foundation/ResMed. I.A. received research support and royalties on continuous positive airway pressure titration patents from Fisher & Paykel. D.J.E. served as a consultant and on an advisory committee for Apnimed; received research support from Bayer and Oventus Medical; and received a senior research fellowship from the National Health and Medical Research Council of Australia. S.J. served as a consultant for Respicardia; and received research support from the NIH for a trial on LOFT-HF. R.N.K. served as a consultant for Respicardia; and served as a speaker for Philips Respironics. R.M. served on an advisory committee for the American Board of Internal Medicine, Merck, and Respicardia; served as a consultant for Respicardia; received research support from the American Heart Association, the NIH, Natus, Philips Respironics, and ResMed; and received royalties from UpToDate. W.J.R. served as a speaker for Bayer Vital, Berlin-Chemie, Bioprojet, Boehringer Ingelheim, Heinen & Löwenstein, Inspire, Novartis, Night Balance, Philips Respironics, ResMed, Vanda Pharma, and Weinman; and received research support from Heinen & Löwenstein and Philips Respironics. V.K.S. served as a consultant for Baker Tilly, Bayer, Jazz Pharmaceutical, GlaxoSmithKline, ResMed, Respicardia, and Roche; and received research support from the NIH, Philips Foundation, and Sleep Number. M.S.B., J.L.F., C.L.J., J.L.M., M.T.N., and S.A.S. reported no commercial or relevant noncommercial interests.
Contributor Information
Collaborators: on behalf of the American Thoracic Society Assembly on Sleep and Respiratory Neurobiology
References
- 1.Javaheri S, Parker TJ, Liming JD, Corbett WS, Nishiyama H, Wexler L, et al. Sleep apnea in 81 ambulatory male patients with stable heart failure: types and their prevalences, consequences, and presentations. Circulation. 1998;97:2154–2159. doi: 10.1161/01.cir.97.21.2154. [DOI] [PubMed] [Google Scholar]
- 2.MacDonald M, Fang J, Pittman SD, White DP, Malhotra A. The current prevalence of sleep disordered breathing in congestive heart failure patients treated with beta-blockers. J Clin Sleep. 2008;4:38–42. [PMC free article] [PubMed] [Google Scholar]
- 3.Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160:1101–1106. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 4.Javaheri S, Shukla R, Zeigler H, Wexler L. Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol. 2007;49:2028–2034. doi: 10.1016/j.jacc.2007.01.084. [DOI] [PubMed] [Google Scholar]
- 5.Oldenburg O, Lamp B, Faber L, Teschler H, Horstkotte D, Töpfer V. Sleep-disordered breathing in patients with symptomatic heart failure: a contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail. 2007;9:251–257. doi: 10.1016/j.ejheart.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Randerath W, Verbraecken J, Andreas S, Arzt M, Bloch KE, Brack T, et al. Definition, discrimination, diagnosis and treatment of central breathing disturbances during sleep. Eur Respir J. 2017;49:1600959. doi: 10.1183/13993003.00959-2016. [DOI] [PubMed] [Google Scholar]
- 7.Aurora RN, Bista SR, Casey KR, Chowdhuri S, Kristo DA, Mallea JM, et al. Updated adaptive servo-ventilation recommendations for the 2012 AASM guideline: “The treatment of central sleep apnea syndromes in adults: practice parameters with an evidence-based literature review and meta-analyses”. J Clin Sleep Med. 2016;12:757–761. doi: 10.5664/jcsm.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dempsey JA, Smith CA. Pathophysiology of human ventilatory control. Eur Respir J. 2014;44:495–512. doi: 10.1183/09031936.00048514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Del Negro CA, Funk GD, Feldman JL. Breathing matters. Nat Rev Neurosci. 2018;19:351–367. doi: 10.1038/s41583-018-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hocker AD, Stokes JA, Powell FL, Huxtable AG. The impact of inflammation on respiratory plasticity. Exp Neurol. 2017;287:243–253. doi: 10.1016/j.expneurol.2016.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behan M, Zabka AG, Mitchell GS. Age and gender effects on serotonin-dependent plasticity in respiratory motor control. Respir Physiol Neurobiol. 2002;131:65–77. doi: 10.1016/s1569-9048(02)00038-1. [DOI] [PubMed] [Google Scholar]
- 12.Javaheri S. A mechanism of central sleep apnea in patients with heart failure. N Engl J Med. 1999;341:949–954. doi: 10.1056/NEJM199909233411304. [DOI] [PubMed] [Google Scholar]
- 13.Solin P, Roebuck T, Johns DP, Walters EH, Naughton MT. Peripheral and central ventilatory responses in central sleep apnea with and without congestive heart failure. Am J Respir Crit Care Med. 2000;162:2194–2200. doi: 10.1164/ajrccm.162.6.2002024. [DOI] [PubMed] [Google Scholar]
- 14.Topor ZL, Johannson L, Kasprzyk J, Remmers JE. Dynamic ventilatory response to CO2 in congestive heart failure patients with and without central sleep apnea. J Appl Physiol (1985) 2001;91:408–416. doi: 10.1152/jappl.2001.91.1.408. [DOI] [PubMed] [Google Scholar]
- 15.Xie A, Skatrud JB, Puleo DS, Rahko PS, Dempsey JA. Apnea-hypopnea threshold for CO2 in patients with congestive heart failure. Am J Respir Crit Care Med. 2002;165:1245–1250. doi: 10.1164/rccm.200110-022OC. [DOI] [PubMed] [Google Scholar]
- 16.Francis DP, Willson K, Davies LC, Coats AJ, Piepoli M. Quantitative general theory for periodic breathing in chronic heart failure and its clinical implications. Circulation. 2000;102:2214–2221. doi: 10.1161/01.cir.102.18.2214. [DOI] [PubMed] [Google Scholar]
- 17.Ponikowski P, Anker SD, Chua TP, Francis D, Banasiak W, Poole-Wilson PA, et al. Oscillatory breathing patterns during wakefulness in patients with chronic heart failure: clinical implications and role of augmented peripheral chemosensitivity. Circulation. 1999;100:2418–2424. doi: 10.1161/01.cir.100.24.2418. [DOI] [PubMed] [Google Scholar]
- 18.Giannoni A, Emdin M, Poletti R, Bramanti F, Prontera C, Piepoli M, et al. Clinical significance of chemosensitivity in chronic heart failure: influence on neurohormonal derangement, Cheyne-Stokes respiration and arrhythmias. Clin Sci (Lond) 2008;114:489–497. doi: 10.1042/CS20070292. [DOI] [PubMed] [Google Scholar]
- 19.Sands SA, Mebrate Y, Edwards BA, Nemati S, Manisty CH, Desai AS, et al. Resonance as the mechanism of daytime periodic breathing in patients with heart failure. Am J Respir Crit Care Med. 2017;195:237–246. doi: 10.1164/rccm.201604-0761OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dempsey JA. Crossing the apnoeic threshold: causes and consequences. Exp Physiol. 2005;90:13–24. doi: 10.1113/expphysiol.2004.028985. [DOI] [PubMed] [Google Scholar]
- 21.Chowdhuri S, Pranathiageswaran S, Loomis-King H, Salloum A, Badr MS. Aging is associated with increased propensity for central apnea during NREM sleep. J Appl Physiol (1985) 2018;124:83–90. doi: 10.1152/japplphysiol.00125.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salloum A, Rowley JA, Mateika JH, Chowdhuri S, Omran Q, Badr MS. Increased propensity for central apnea in patients with obstructive sleep apnea: effect of nasal continuous positive airway pressure. Am J Respir Crit Care Med. 2010;181:189–193. doi: 10.1164/rccm.200810-1658OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou XS, Shahabuddin S, Zahn BR, Babcock MA, Badr MS. Effect of gender on the development of hypocapnic apnea/hypopnea during NREM sleep. J Appl Physiol (1985) 2000;89:192–199. doi: 10.1152/jappl.2000.89.1.192. [DOI] [PubMed] [Google Scholar]
- 24.Zhou XS, Rowley JA, Demirovic F, Diamond MP, Badr MS. Effect of testosterone on the apneic threshold in women during NREM sleep. J Appl Physiol (1985) 2003;94:101–107. doi: 10.1152/japplphysiol.00264.2002. [DOI] [PubMed] [Google Scholar]
- 25.Mateika JH, Omran Q, Rowley JA, Zhou XS, Diamond MP, Badr MS. Treatment with leuprolide acetate decreases the threshold of the ventilatory response to carbon dioxide in healthy males. J Physiol. 2004;561:637–646. doi: 10.1113/jphysiol.2004.071811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chowdhuri S, Bascom A, Mohan D, Diamond MP, Badr MS. Testosterone conversion blockade increases breathing stability in healthy men during NREM sleep. Sleep (Basel) 2013;36:1793–1798. doi: 10.5665/sleep.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chowdhuri S, Shanidze I, Pierchala L, Belen D, Mateika JH, Badr MS. Effect of episodic hypoxia on the susceptibility to hypocapnic central apnea during NREM sleep. J Appl Physiol (1985) 2010;108:369–377. doi: 10.1152/japplphysiol.00308.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chowdhuri S, Sinha P, Pranathiageswaran S, Badr MS. Sustained hyperoxia stabilizes breathing in healthy individuals during NREM sleep. J Appl Physiol (1985) 2010;109:1378–1383. doi: 10.1152/japplphysiol.00453.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lloyd TC., Jr Effect of increased left atrial pressure on breathing frequency in anesthetized dog. J Appl Physiol (1985) 1990;69:1973–1980. doi: 10.1152/jappl.1990.69.6.1973. [DOI] [PubMed] [Google Scholar]
- 30.Lloyd TC., Jr Breathing response to lung congestion with and without left heart distension. J Appl Physiol (1985) 1988;65:131–136. doi: 10.1152/jappl.1988.65.1.131. [DOI] [PubMed] [Google Scholar]
- 31.Szollosi I, Thompson BR, Krum H, Kaye DM, Naughton MT. Impaired pulmonary diffusing capacity and hypoxia in heart failure correlates with central sleep apnea severity. Chest. 2008;134:67–72. doi: 10.1378/chest.07-1487. [DOI] [PubMed] [Google Scholar]
- 32.White LH, Bradley TD. Role of nocturnal rostral fluid shift in the pathogenesis of obstructive and central sleep apnoea. J Physiol. 2013;591:1179–1193. doi: 10.1113/jphysiol.2012.245159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mortara A, Sleight P, Pinna GD, Maestri R, Capomolla S, Febo O, et al. Association between hemodynamic impairment and Cheyne-Stokes respiration and periodic breathing in chronic stable congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1999;84:900–904. doi: 10.1016/s0002-9149(99)00462-2. [DOI] [PubMed] [Google Scholar]
- 34.Hall MJ, Xie A, Rutherford R, Ando S, Floras JS, Bradley TD. Cycle length of periodic breathing in patients with and without heart failure. Am J Respir Crit Care Med. 1996;154:376–381. doi: 10.1164/ajrccm.154.2.8756809. [DOI] [PubMed] [Google Scholar]
- 35.Solin P, Roebuck T, Swieca J, Walters EH, Naughton MT. Effects of cardiac dysfunction on non-hypercapnic central sleep apnea. Chest. 1998;113:104–110. doi: 10.1378/chest.113.1.104. [DOI] [PubMed] [Google Scholar]
- 36.Stanchina ML, Ellison K, Malhotra A, Anderson M, Kirk M, Benser ME, et al. The impact of cardiac resynchronization therapy on obstructive sleep apnea in heart failure patients: a pilot study. Chest. 2007;132:433–439. doi: 10.1378/chest.06-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orr JE, Auger WR, DeYoung PN, Kim NH, Malhotra A, Owens RL. Usefulness of low cardiac index to predict sleep-disordered breathing in chronic thromboembolic pulmonary hypertension. Am J Cardiol. 2016;117:1001–1005. doi: 10.1016/j.amjcard.2015.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oldenburg O, Bitter T, Wiemer M, Langer C, Horstkotte D, Piper C. Pulmonary capillary wedge pressure and pulmonary arterial pressure in heart failure patients with sleep-disordered breathing. Sleep Med. 2009;10:726–730. doi: 10.1016/j.sleep.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 39.Sinha AM, Skobel EC, Breithardt OA, Norra C, Markus KU, Breuer C, et al. Cardiac resynchronization therapy improves central sleep apnea and Cheyne-Stokes respiration in patients with chronic heart failure. J Am Coll Cardiol. 2004;44:68–71. doi: 10.1016/j.jacc.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 40.Olson TP, Frantz RP, Snyder EM, O’Malley KA, Beck KC, Johnson BD. Effects of acute changes in pulmonary wedge pressure on periodic breathing at rest in heart failure patients. Am Heart J. 2007;153:104, e1–e7. doi: 10.1016/j.ahj.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy RM, Shah RV, Malhotra R, Pappagianopoulos PP, Hough SS, Systrom DM, et al. Exercise oscillatory ventilation in systolic heart failure: an indicator of impaired hemodynamic response to exercise. Circulation. 2011;124:1442–1451. doi: 10.1161/CIRCULATIONAHA.111.024141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bascom AT, Sankari A, Badr MS. Spinal cord injury is associated with enhanced peripheral chemoreflex sensitivity. Physiol Rep. 2016;4:e12948. doi: 10.14814/phy2.12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sankari A, Bascom AT, Chowdhuri S, Badr MS. Tetraplegia is a risk factor for central sleep apnea. J Appl Physiol (1985) 2014;116:345–353. doi: 10.1152/japplphysiol.00731.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eckert DJ, Owens RL, Kehlmann GB, Wellman A, Rahangdale S, Yim-Yeh S, et al. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci (Lond) 2011;120:505–514. doi: 10.1042/CS20100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eckert DJ, Malhotra A, Wellman A, White DP. Trazodone increases the respiratory arousal threshold in patients with obstructive sleep apnea and a low arousal threshold. Sleep (Basel) 2014;37:811–819. doi: 10.5665/sleep.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edwards BA, Sands SA, Owens RL, Eckert DJ, Landry S, White DP, et al. The combination of supplemental oxygen and a hypnotic markedly improves obstructive sleep apnea in patients with a mild to moderate upper airway collapsibility. Sleep (Basel) 2016;39:1973–1983. doi: 10.5665/sleep.6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eckert DJ, Malhotra A, Jordan AS. Mechanisms of apnea. Prog Cardiovasc Dis. 2009;51:313–323. doi: 10.1016/j.pcad.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Badr MS, Toiber F, Skatrud JB, Dempsey J. Pharyngeal narrowing/occlusion during central sleep apnea. J Appl Physiol (1985) 1995;78:1806–1815. doi: 10.1152/jappl.1995.78.5.1806. [DOI] [PubMed] [Google Scholar]
- 49.Badr MS. Effect of ventilatory drive on upper airway patency in humans during NREM sleep. Respir Physiol. 1996;103:1–10. doi: 10.1016/0034-5687(95)00079-8. [DOI] [PubMed] [Google Scholar]
- 50.Hudgel DW, Chapman KR, Faulks C, Hendricks C. Changes in inspiratory muscle electrical activity and upper airway resistance during periodic breathing induced by hypoxia during sleep. Am Rev Respir Dis. 1987;135:899–906. doi: 10.1164/arrd.1987.135.4.899. [DOI] [PubMed] [Google Scholar]
- 51.Warner G, Skatrud JB, Dempsey JA. Effect of hypoxia-induced periodic breathing on upper airway obstruction during sleep. J Appl Physiol (1985) 1987;62:2201–2211. doi: 10.1152/jappl.1987.62.6.2201. [DOI] [PubMed] [Google Scholar]
- 52.Morrell MJ, Arabi Y, Zahn BR, Meyer KC, Skatrud JB, Badr MS. Effect of surfactant on pharyngeal mechanics in sleeping humans: implications for sleep apnoea. Eur Respir J. 2002;20:451–457. doi: 10.1183/09031936.02.00273702. [DOI] [PubMed] [Google Scholar]
- 53.Sankri-Tarbichi AG, Rowley JA, Badr MS. Inhibition of ventilatory motor output increases expiratory retro palatal compliance during sleep. Respir Physiol Neurobiol. 2011;176:136–143. doi: 10.1016/j.resp.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sankri-Tarbichi AG, Rowley JA, Badr MS. Expiratory pharyngeal narrowing during central hypocapnic hypopnea. Am J Respir Crit Care Med. 2009;179:313–319. doi: 10.1164/rccm.200805-741OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bucca CB, Brussino L, Battisti A, Mutani R, Rolla G, Mangiardi L, et al. Diuretics in obstructive sleep apnea with diastolic heart failure. Chest. 2007;132:440–446. doi: 10.1378/chest.07-0311. [DOI] [PubMed] [Google Scholar]
- 56.Javaheri S, Brown LK, Khayat RN. Update on apneas of heart failure with reduced ejection fraction: emphasis on the physiology of treatment. Part 2: central sleep apnea. Chest. 2020;157:1637–1646. doi: 10.1016/j.chest.2019.12.020. [DOI] [PubMed] [Google Scholar]
- 57.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–148. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 58.Donovan LM, Kapur VK. Prevalence and characteristics of central compared to obstructive sleep apnea: analyses from the sleep heart health study cohort. Sleep (Basel) 2016;39:1353–1359. doi: 10.5665/sleep.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kirkness JP, Schwartz AR, Schneider H, Punjabi NM, Maly JJ, Laffan AM, et al. Contribution of male sex, age, and obesity to mechanical instability of the upper airway during sleep. J Appl Physiol (1985) 2008;104:1618–1624. doi: 10.1152/japplphysiol.00045.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharma G, Goodwin J. Effect of aging on respiratory system physiology and immunology. Clin Interv Aging. 2006;1:253–260. doi: 10.2147/ciia.2006.1.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, Vela-Bueno A, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163:608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 62.Rowley JA, Zhou XS, Diamond MP, Badr MS. The determinants of the apnea threshold during NREM sleep in normal subjects. Sleep. 2006;29:95–103. doi: 10.1093/sleep/29.1.95. [DOI] [PubMed] [Google Scholar]
- 63.Arzt M, Young T, Finn L, Skatrud JB, Ryan CM, Newton GE, et al. Sleepiness and sleep in patients with both systolic heart failure and obstructive sleep apnea. Arch Intern Med. 2006;166:1716–1722. doi: 10.1001/archinte.166.16.1716. [DOI] [PubMed] [Google Scholar]
- 64.Ferrier K, Campbell A, Yee B, Richards M, O’Meeghan T, Weatherall M, et al. Sleep-disordered breathing occurs frequently in stable outpatients with congestive heart failure. Chest. 2005;128:2116–2122. doi: 10.1378/chest.128.4.2116. [DOI] [PubMed] [Google Scholar]
- 65.Taranto Montemurro L, Floras JS, Millar PJ, Kasai T, Gabriel JM, Spaak J, et al. Inverse relationship of subjective daytime sleepiness to sympathetic activity in patients with heart failure and obstructive sleep apnea. Chest. 2012;142:1222–1228. doi: 10.1378/chest.11-2963. [DOI] [PubMed] [Google Scholar]
- 66.Sharma S, Mather P, Efird JT, Kahn D, Cheema M, Rubin S, et al. Photoplethysmographic signal to screen sleep-disordered breathing in hospitalized heart failure patients: feasibility of a prospective clinical pathway. JACC Heart Fail. 2015;3:725–731. doi: 10.1016/j.jchf.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 67.Solin P, Bergin P, Richardson M, Kaye DM, Walters EH, Naughton MT. Influence of pulmonary capillary wedge pressure on central apnea in heart failure. Circulation. 1999;99:1574–1579. doi: 10.1161/01.cir.99.12.1574. [DOI] [PubMed] [Google Scholar]
- 68.Padeletti M, Green P, Mooney AM, Basner RC, Mancini DM. Sleep disordered breathing in patients with acutely decompensated heart failure. Sleep Med. 2009;10:353–360. doi: 10.1016/j.sleep.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 69.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the sleep apnea definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Terrill PI, Edwards BA, Nemati S, Butler JP, Owens RL, Eckert DJ, et al. Quantifying the ventilatory control contribution to sleep apnoea using polysomnography. Eur Respir J. 2015;45:408–418. doi: 10.1183/09031936.00062914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sands SA, Terrill PI, Edwards BA, Taranto Montemurro L, Azarbarzin A, Marques M, et al. Quantifying the arousal threshold using polysomnography in obstructive sleep apnea. Sleep (Basel) 2018;41:zsx183. doi: 10.1093/sleep/zsx183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Osman AM, Carter SG, Carberry JC, Eckert DJ. Obstructive sleep apnea: current perspectives. Nat Sci Sleep. 2018;10:21–34. doi: 10.2147/NSS.S124657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Javed F, Tamisier R, Pepin J-L, Cowie MR, Wegscheider K, Angermann C, et al. Association of serious adverse events with Cheyne-Stokes respiration characteristics in patients with systolic heart failure and central sleep apnoea: a SERVE-Heart Failure substudy analysis. Respirology. 2020;25:305–311. doi: 10.1111/resp.13613. [DOI] [PubMed] [Google Scholar]
- 74.Sands SA, Edwards BA, Kee K, Turton A, Skuza EM, Roebuck T, et al. Loop gain as a means to predict a positive airway pressure suppression of Cheyne-Stokes respiration in patients with heart failure. Am J Respir Crit Care Med. 2011;184:1067–1075. doi: 10.1164/rccm.201103-0577OC. [DOI] [PubMed] [Google Scholar]
- 75.Mann DL, Terrill PI, Azarbarzin A, Mariani S, Franciosini A, Camassa A, et al. Quantifying the magnitude of pharyngeal obstruction during sleep using airflow shape. Eur Respir J. 2019;54:1802262. doi: 10.1183/13993003.02262-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Messineo L, Taranto-Montemurro L, Azarbarzin A, Oliveira Marques MD, Calianese N, White DP, et al. Breath-holding as a means to estimate the loop gain contribution to obstructive sleep apnoea. J Physiol. 2018;596:4043–4056. doi: 10.1113/JP276206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nava-Guerra L, Edwards BA, Terrill PI, Sands SA, Amin RS, Kemp JS, et al. Quantifying ventilatory control stability from spontaneous sigh responses during sleep: a comparison of two approaches. Physiol Meas. 2018;39:114005. doi: 10.1088/1361-6579/aae7a9. [DOI] [PubMed] [Google Scholar]
- 78.Azarbarzin A, Sands SA, Stone KL, Taranto-Montemurro L, Messineo L, Terrill PI, et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: the Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur Heart J. 2019;40:1149–1157. doi: 10.1093/eurheartj/ehy624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Corrà U, Pistono M, Mezzani A, Braghiroli A, Giordano A, Lanfranchi P, et al. Sleep and exertional periodic breathing in chronic heart failure: prognostic importance and interdependence. Circulation. 2006;113:44–50. doi: 10.1161/CIRCULATIONAHA.105.543173. [DOI] [PubMed] [Google Scholar]
- 80.Javaheri S. Effects of continuous positive airway pressure on sleep apnea and ventricular irritability in patients with heart failure. Circulation. 2000;101:392–397. doi: 10.1161/01.cir.101.4.392. [DOI] [PubMed] [Google Scholar]
- 81.Arzt M, Floras JS, Logan AG, Kimoff RJ, Series F, Morrison D, et al. CANPAP Investigators. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP) Circulation. 2007;115:3173–3180. doi: 10.1161/CIRCULATIONAHA.106.683482. [DOI] [PubMed] [Google Scholar]
- 82.Naughton MT, Benard DC, Liu PP, Rutherford R, Rankin F, Bradley TD. Effects of nasal CPAP on sympathetic activity in patients with heart failure and central sleep apnea. Am J Respir Crit Care Med. 1995;152:473–479. doi: 10.1164/ajrccm.152.2.7633695. [DOI] [PubMed] [Google Scholar]
- 83.Mansfield DR, Gollogly NC, Kaye DM, Richardson M, Bergin P, Naughton MT. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med. 2004;169:361–366. doi: 10.1164/rccm.200306-752OC. [DOI] [PubMed] [Google Scholar]
- 84.Naughton MT. Cheyne-Stokes respiration: friend or foe? Thorax. 2012;67:357–360. doi: 10.1136/thoraxjnl-2011-200927. [DOI] [PubMed] [Google Scholar]
- 85.Perger E, Inami T, Lyons OD, Alshaer H, Smith S, Floras JS, et al. ADVENT-HF Investigators. Distinct patterns of hyperpnea during Cheyne-Stokes respiration: implication for cardiac function in patients with heart failure. J Clin Sleep Med. 2017;13:1235–1241. doi: 10.5664/jcsm.6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Inami T, Kasai T, Yumino D, Perger E, Alshaer H, Hummel R, et al. Relationship of stroke volume to different patterns of Cheyne-Stokes respiration in heart failure. Sleep. 2019;42:zsy262. doi: 10.1093/sleep/zsy262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Harun NS, Leet A, Naughton MT. Improvement in sleep-disordered breathing after insertion of left ventricular assist device. Ann Am Thorac Soc. 2013;10:272–273. doi: 10.1513/AnnalsATS.201304-075SF. [DOI] [PubMed] [Google Scholar]
- 88.Mansfield DR, Solin P, Roebuck T, Bergin P, Kaye DM, Naughton MT. The effect of successful heart transplant treatment of heart failure on central sleep apnea. Chest. 2003;124:1675–1681. doi: 10.1378/chest.124.5.1675. [DOI] [PubMed] [Google Scholar]
- 89.Johnson BD, Joyner MJ. Carotid body denervation: too soon to get breathless about heart failure? J Am Coll Cardiol. 2013;62:2431–2432. doi: 10.1016/j.jacc.2013.08.718. [DOI] [PubMed] [Google Scholar]
- 90.Mendelson M, Inami T, Lyons O, Alshaer H, Marzolini S, Oh P, et al. Long-term effects of cardiac rehabilitation on sleep apnea severity in patients with coronary artery disease. J Clin Sleep Med. 2020;16:65–71. doi: 10.5664/jcsm.8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bradley TD, Logan AG, Kimoff RJ, Sériès F, Morrison D, Ferguson K, et al. CANPAP Investigators. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353:2025–2033. doi: 10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 92.Cowie MR, Woehrle H, Wegscheider K, Angermann C, d’Ortho M-P, Erdmann E, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373:1095–1105. doi: 10.1056/NEJMoa1506459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.O’Connor CM, Whellan DJ, Fiuzat M, Punjabi NM, Tasissa G, Anstrom KJ, et al. Cardiovascular outcomes with minute ventilation-targeted adaptive servo-ventilation therapy in heart failure: the CAT-HF trial. J Am Coll Cardiol. 2017;69:1577–1587. doi: 10.1016/j.jacc.2017.01.041. [DOI] [PubMed] [Google Scholar]
- 94.Bradley TD, Floras JS. Adaptive servo-ventilation and the treatment of central sleep apnea in heart failure: let’s not throw the baby out with the bathwater. Am J Respir Crit Care Med. 2016;193:357–359. doi: 10.1164/rccm.201511-2198ED. [DOI] [PubMed] [Google Scholar]
- 95.Javaheri S, Brown LK, Randerath W, Khayat R. SERVE-HF: more questions than answers. Chest. 2016;149:900–904. doi: 10.1016/j.chest.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 96.Woehrle H, Cowie MR, Eulenburg C, Suling A, Angermann C, d’Ortho MP, et al. Adaptive servo ventilation for central sleep apnoea in heart failure: SERVE-HF on-treatment analysis. Eur Respir J. 2017;50:1601692. doi: 10.1183/13993003.01692-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Eulenburg C, Wegscheider K, Woehrle H, Angermann C, d’Ortho MP, Erdmann E, et al. Mechanisms underlying increased mortality risk in patients with heart failure and reduced ejection fraction randomly assigned to adaptive servoventilation in the SERVE-HF study: results of a secondary multistate modelling analysis. Lancet Respir Med. 2016;4:873–881. doi: 10.1016/S2213-2600(16)30244-2. [DOI] [PubMed] [Google Scholar]
- 98.Cowie MR, Woehrle H, Wegscheider K, Vettorazzi E, Lezius S, Koenig W, et al. Adaptive servo-ventilation for central sleep apnoea in systolic heart failure: results of the major substudy of SERVE-HF. Eur J Heart Fail. 2018;20:536–544. doi: 10.1002/ejhf.1048. [DOI] [PubMed] [Google Scholar]
- 99.Ferreira JP, Duarte K, Woehrle H, Cowie MR, Angermann C, d’Ortho M-P, et al. Bioprofiles and mechanistic pathways associated with Cheyne-Stokes respiration: insights from the SERVE-HF trial. Clin Res Cardiol. 2020;109:881–891. doi: 10.1007/s00392-019-01578-9. [DOI] [PubMed] [Google Scholar]
- 100.Costanzo MR, Ponikowski P, Javaheri S, Augostini R, Goldberg L, Holcomb R, et al. Remedé System Pivotal Trial Study Group. Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial. Lancet. 2016;388:974–982. doi: 10.1016/S0140-6736(16)30961-8. [DOI] [PubMed] [Google Scholar]
- 101.Fox H, Oldenburg O, Javaheri S, Ponikowski P, Augostini R, Goldberg LR, et al. Long-term efficacy and safety of phrenic nerve stimulation for the treatment of central sleep apnea. Sleep. 2019;42:zsz158. doi: 10.1093/sleep/zsz158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lorenzi-Filho G, Rankin F, Bies I, Douglas Bradley T. Effects of inhaled carbon dioxide and oxygen on cheyne-stokes respiration in patients with heart failure. Am J Respir Crit Care Med. 1999;159:1490–1498. doi: 10.1164/ajrccm.159.5.9810040. [DOI] [PubMed] [Google Scholar]
- 103.Steens RD, Millar TW, Su X, Biberdorf D, Buckle P, Ahmed M, et al. Effect of inhaled 3% CO2 on Cheyne-Stokes respiration in congestive heart failure. Sleep. 1994;17:61–68. doi: 10.1093/sleep/17.1.61. [DOI] [PubMed] [Google Scholar]
- 104.Giannoni A, Baruah R, Willson K, Mebrate Y, Mayet J, Emdin M, et al. Real-time dynamic carbon dioxide administration: a novel treatment strategy for stabilization of periodic breathing with potential application to central sleep apnea. J Am Coll Cardiol. 2010;56:1832–1837. doi: 10.1016/j.jacc.2010.05.053. [DOI] [PubMed] [Google Scholar]
- 105.Khayat RN, Xie A, Patel AK, Kaminski A, Skatrud JB. Cardiorespiratory effects of added dead space in patients with heart failure and central sleep apnea. Chest. 2003;123:1551–1560. doi: 10.1378/chest.123.5.1551. [DOI] [PubMed] [Google Scholar]
- 106.Gilmartin G, McGeehan B, Vigneault K, Daly RW, Manento M, Weiss JW, et al. Treatment of positive airway pressure treatment-associated respiratory instability with enhanced expiratory rebreathing space (EERS) J Clin Sleep Med. 2010;6:529–538. [PMC free article] [PubMed] [Google Scholar]
- 107.Sands SA, Edwards BA, Kee K, Stuart-Andrews C, Skuza EM, Roebuck T, et al. Control theory prediction of resolved Cheyne-Stokes respiration in heart failure. Eur Respir J. 2016;48:1351–1359. doi: 10.1183/13993003.00615-2016. [DOI] [PubMed] [Google Scholar]
- 108.Javaheri S, Parker TJ, Wexler L, Liming JD, Lindower P, Roselle GA. Effect of theophylline on sleep-disordered breathing in heart failure. N Engl J Med. 1996;335:562–567. doi: 10.1056/NEJM199608223350805. [DOI] [PubMed] [Google Scholar]
- 109.Liu HM, Chiang IJ, Kuo KN, Liou CM, Chen C. The effect of acetazolamide on sleep apnea at high altitude: a systematic review and meta-analysis. Ther Adv Respir Dis. 2017;11:20–29. doi: 10.1177/1753465816677006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schmickl CN, Owens RL, Orr JE, Edwards BA, Malhotra A. Side effects of acetazolamide: a systematic review and meta-analysis assessing overall risk and dose dependence. BMJ Open Respir Res. 2020;7:e000557. doi: 10.1136/bmjresp-2020-000557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.DeBacker WA, Verbraecken J, Willemen M, Wittesaele W, DeCock W, Van deHeyning P. Central apnea index decreases after prolonged treatment with acetazolamide. Am J Respir Crit Care Med. 1995;151:87–91. doi: 10.1164/ajrccm.151.1.7812578. [DOI] [PubMed] [Google Scholar]
- 112.Javaheri S. Acetazolamide improves central sleep apnea in heart failure: a double-blind, prospective study. Am J Respir Crit Care Med. 2006;173:234–237. doi: 10.1164/rccm.200507-1035OC. [DOI] [PubMed] [Google Scholar]
- 113.Fontana M, Emdin M, Giannoni A, Iudice G, Baruah R, Passino C. Effect of acetazolamide on chemosensitivity, Cheyne-Stokes respiration, and response to effort in patients with heart failure. Am J Cardiol. 2011;107:1675–1680. doi: 10.1016/j.amjcard.2011.01.060. [DOI] [PubMed] [Google Scholar]
- 114.Edwards BA, Sands SA, Eckert DJ, White DP, Butler JP, Owens RL, et al. Acetazolamide improves loop gain but not the other physiological traits causing obstructive sleep apnoea. J Physiol. 2012;590:1199–1211. doi: 10.1113/jphysiol.2011.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Aaslid R. Cerebral autoregulation and vasomotor reactivity. Front Neurol Neurosci. 2006;21:216–228. doi: 10.1159/000092434. [DOI] [PubMed] [Google Scholar]
- 116.Ginter G, Sankari A, Eshraghi M, Obiakor H, Yarandi H, Chowdhuri S, et al. Effect of acetazolamide on susceptibility to central sleep apnea in chronic spinal cord injury. J Appl Physiol (1985) 2020;128:960–966. doi: 10.1152/japplphysiol.00532.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Quadri S, Drake C, Hudgel DW. Improvement of idiopathic central sleep apnea with zolpidem. J Clin Sleep Med. 2009;5:122–129. [PMC free article] [PubMed] [Google Scholar]
- 118.Mendelson WB, Martin JV, Rapoport DM. Effects of buspirone on sleep and respiration. Am Rev Respir Dis. 1990;141:1527–1530. doi: 10.1164/ajrccm/141.6.1527. [DOI] [PubMed] [Google Scholar]
- 119.Yamauchi M, Dostal J, Kimura H, Strohl KP. Effects of buspirone on posthypoxic ventilatory behavior in the C57BL/6J and A/J mouse strains. J Appl Physiol (1985) 2008;105:518–526. doi: 10.1152/japplphysiol.00069.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Andaku DK, Mercadante MT, Schwartzman JS. Buspirone in Rett syndrome respiratory dysfunction. Brain Dev. 2005;27:437–438. doi: 10.1016/j.braindev.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 121.El-Khatib MF, Kiwan RA, Jamaleddine GW. Buspirone treatment for apneustic breathing in brain stem infarct. Respir Care. 2003;48:956–958. [PubMed] [Google Scholar]
- 122.Mendelson WB, Maczaj M, Holt J. Buspirone administration to sleep apnea patients. J Clin Psychopharmacol. 1991;11:71–72. doi: 10.1097/00004714-199102000-00019. [DOI] [PubMed] [Google Scholar]
- 123.Marcus NJ, Del Rio R, Ding Y, Schultz HD. KLF2 mediates enhanced chemoreflex sensitivity, disordered breathing and autonomic dysregulation in heart failure. J Physiol. 2018;596:3171–3185. doi: 10.1113/JP273805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gosselin LE, Barkley JE, Spencer MJ, McCormick KM, Farkas GA. Ventilatory dysfunction in mdx mice: impact of tumor necrosis factor-alpha deletion. Muscle Nerve. 2003;28:336–343. doi: 10.1002/mus.10431. [DOI] [PubMed] [Google Scholar]
- 125.Huxtable AG, Smith SM, Peterson TJ, Watters JJ, Mitchell GS. Intermittent hypoxia-induced spinal inflammation impairs respiratory motor plasticity by a spinal p38 MAP kinase-dependent mechanism. J Neurosci. 2015;35:6871–6880. doi: 10.1523/JNEUROSCI.4539-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Peng YJ, Zhang X, Gridina A, Chupikova I, McCormick DL, Thomas RJ, et al. Complementary roles of gasotransmitters CO and H2S in sleep apnea. Proc Natl Acad Sci U S A. 2017;114:1413–1418. doi: 10.1073/pnas.1620717114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ford AP, Undem BJ, Birder LA, Grundy D, Pijacka W, Paton JF. P2X3 receptors and sensitization of autonomic reflexes. Auton Neurosci. 2015;191:16–24. doi: 10.1016/j.autneu.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 128.Niewinski P, Janczak D, Rucinski A, Tubek S, Engelman ZJ, Piesiak P, et al. Carotid body resection for sympathetic modulation in systolic heart failure: results from first-in-man study. Eur J Heart Fail. 2017;19:391–400. doi: 10.1002/ejhf.641. [DOI] [PubMed] [Google Scholar]
- 129.Peacock E, Krousel-Wood M. Adherence to antihypertensive therapy. Med Clin North Am. 2017;101:229–245. doi: 10.1016/j.mcna.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011;86:304–314. doi: 10.4065/mcp.2010.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rotenberg BW, Murariu D, Pang KP. Trends in CPAP adherence over twenty years of data collection: a flattened curve. J Otolaryngol Head Neck Surg. 2016;45:43. doi: 10.1186/s40463-016-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pepin J-L, Woehrle H, Liu D, Shao S, Armitstead JP, Cistulli PA, et al. Adherence to positive airway therapy after switching from CPAP to ASV: a big data analysis. J Clin Sleep Med. 2018;14:57–63. doi: 10.5664/jcsm.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, et al. SAVE Investigators and Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 134.Perger E, Lyons OD, Inami T, Smith S, Floras JS, Logan AG, et al. ADVENT-HF Investigators; ADVENT-HF Trial Executive Committee; ADVENT-HF Steering Committee; ADVENT-HF Data and Safety Monitoring Committee; ADVENT-HF Event Adjudication Committee; ADVENT-HF Investigators. Predictors of 1-year compliance with adaptive servoventilation in patients with heart failure and sleep disordered breathing: preliminary data from the ADVENT-HF trial. Eur Respir J. 2019;53:1801626. doi: 10.1183/13993003.01626-2018. [DOI] [PubMed] [Google Scholar]
- 135.Bordier P, Lataste A, Hofmann P, Robert F, Bourenane G. Nocturnal oxygen therapy in patients with chronic heart failure and sleep apnea: a systematic review. Sleep Med. 2016;17:149–157. doi: 10.1016/j.sleep.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 136.Gottlieb DJ, Punjabi NM, Mehra R, Patel SR, Quan SF, Babineau DC, et al. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med. 2014;370:2276–2285. doi: 10.1056/NEJMoa1306766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Askland K, Wright L, Wozniak DR, Emmanuel T, Caston J, Smith I. Educational, supportive and behavioural interventions to improve usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea. Cochrane Database Syst Rev. 2020;4:CD007736. doi: 10.1002/14651858.CD007736.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Duran DG, Pérez-Stable EJ. Novel approaches to advance minority health and health disparities research. Am J Public Health. 2019;109:S8–S10. doi: 10.2105/AJPH.2018.304931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Blanc J, Nunes J, Williams N, Robbins R, Seixas AA, Jean-Louis G. Sleep health equity. In: Grandner MA, editor. Sleep and health. Cambridge, MA: Academic Press; 2019. pp. 473–480. [Google Scholar]
- 140.Jackson CL, Walker JR, Brown MK, Das R, Jones NL. A workshop report on the causes and consequences of sleep health disparities. Sleep. 2020;43:zsaa037. doi: 10.1093/sleep/zsaa037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jackson CL, Redline S, Emmons KM. Sleep as a potential fundamental contributor to disparities in cardiovascular health. Annu Rev Public Health. 2015;36:417–440. doi: 10.1146/annurev-publhealth-031914-122838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Johnson DA, Jackson CL, Williams NJ, Alcántara C. Are sleep patterns influenced by race/ethnicity: a marker of relative advantage or disadvantage? Evidence to date. Nat Sci Sleep. 2019;11:79–95. doi: 10.2147/NSS.S169312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bibbins-Domingo K, Pletcher MJ, Lin F, Vittinghoff E, Gardin JM, Arynchyn A, et al. Racial differences in incident heart failure among young adults. N Engl J Med. 2009;360:1179–1190. doi: 10.1056/NEJMoa0807265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bahrami H, Kronmal R, Bluemke DA, Olson J, Shea S, Liu K, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168:2138–2145. doi: 10.1001/archinte.168.19.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Eckert DJ, Butler JE.Section 3, chapter 16: respiratory physiology: understanding the control of ventilation Kryger M, Roth T, Dement WC.Principles and practice of sleep medicine 6th ed.Philadelphia, PA: Elsevier; 2017167–173, e4.. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


