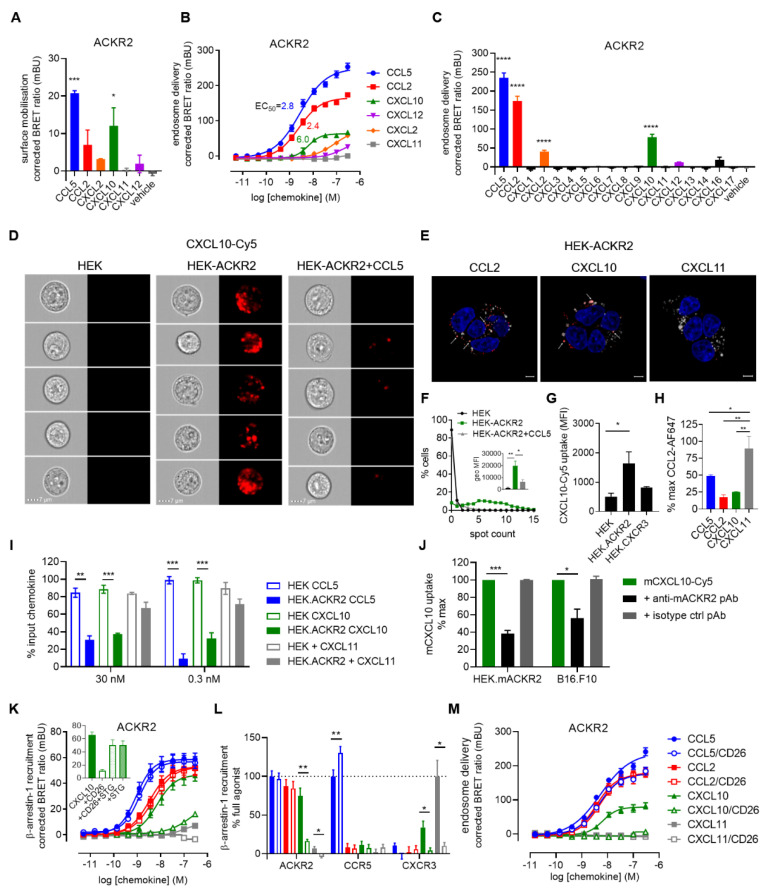
Figure 2.
CXCL10 scavenging by ACKR2. (A) ACKR2 mobilisation to the plasma membrane in response to chemokines (100 nM) monitored by NanoBRET-based assay. (B,C) β-arrestin-1/ACKR2 complex delivery to the early endosomes in response to the CXC chemokines CXCL2, CXCL10 and CXCL12 (B) or the 16 human CXC chemokines (100 nM) (C) monitored by NanoBRET-based assay. CCL2 and CCL5 were used as positive control chemokines. (D–F) Uptake of fluorescently labelled CXCL10 by ACKR2-expressing cells visualized by imaging flow cytometry (D,F) and confocal microscopy (E). (D) HEK, HEK-ACKR2 or HEK-ACKR2 cells pre-treated with CCL5 at saturating concentration (200 nM) were stimulated for 45 min at 37 °C with 100 nM (Cy5)-labelled CXCL10 (CXCL10-Cy5, red channel). Five representative cells for each condition are shown (10,000 events recorded). Scale bar: 7 µm. (F) Percentage of cells from (D) with a given number of distinguishable vesicle-like structures (spots), as well as the geometrical mean fluorescence intensity (MFI) for the red channel were determined (inset). Data shown are representative of three independent experiments and for inset, mean ± SEM of three independent experiments. (E) Cellular localization of Cy5-labelled chemokine (red) following HEK-ACKR2 stimulation (100 nM) for 2 h monitored by fluorescent confocal microscopy. Lysosomes and nucleic DNA were stained using LysoTracker™ Red DND-99 (white) and Hoechst 33342 (blue), respectively. Pictures are representative of 12 acquired images from three independent experiments. Scale bar: 5 µm. Arrows highlight colocalization of Lysotracker and chemokine-Cy5 signal. (G) Uptake of Cy5-labelled chemokine (100 nM) by HEK cells transfected or not with equal amounts of ACKR2 or CXCR3 vectors analysed by imaging flow cytometry as described in (D). (H) Binding competition between Alexa Fluor 647-labelled CCL2 (100 ng/mL) and unlabelled chemokines (100 nM) in HEK-ACKR2 analysed by imaging flow cytometry. (I) ACKR2-mediated depletion of extracellular CXCL10 monitored by ELISA. Chemokines in the supernatant of HEK293T cells expressing or not ACKR2 were quantified after 8 h stimulation, and expressed as percentage of the input concentrations (30 nM and 0.3 nM). CCL5 and CXCL11 were used as positive and negative controls, respectively. Data points represent mean ± SEM of three independent experiments. (J) Inhibition of mACKR2-mediated mCXCL10 uptake by neutralizing antibodies. Cy5-labelled mouse CXCL10 (mCXCL10-Cy5) (100 nM) was incubated with HEK-mACKR2 or B16.F10 in the presence of mACKR2-specific polyclonal antibody (Ab1656) or corresponding isotype control (Ab37373) for 45 min at 37 °C and analysed by flow cytometry. (K–M) Impact of chemokine N-terminal processing by dipeptidyl peptidase 4 (DPP4/CD26) on the activation of ACKR2 and related receptors CXCR3 and CCR5 and ACKR2 delivery to the endosomes. (K,L) β-arrestin-1 recruitment to ACKR2 by processed chemokines monitored by NanoBRET. (L) Comparison of the impact of N-terminal processing on the ability of CXC and CC chemokines (100 nM) to induce β-arrestin-1 recruitment to ACKR2, CXCR3 and CCR5. (Inset) Comparison of ACKR2 activity induced by unprocessed CXCL10 or CXCL10 treated with CD26 in the presence or absence of its specific inhibitor, sitagliptin (STG) (10 µM) or with STG alone, demonstrating no interference between CD26 and the ACKR2-CXCL10 interaction. (M) β-arrestin-1/ACKR2 complex delivery to the early endosomes in response to processed chemokines monitored by NanoBRET. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 by one-way ANOVA with Dunnet (A,C) and Bonferroni (H) post hoc tests or repeated measures one-way ANOVA with Bonferroni post hoc test (J) and two-tailed unpaired Student’s t-test (I).