Abstract
Prosocial behaviors are hypothesized to require socio-cognitive and empathic abilities—engaging brain regions attributed to the mentalizing and empathy brain networks. Here, we tested this hypothesis with a coordinate-based meta-analysis of 600 neuroimaging studies on prosociality, mentalizing and empathy (~12,000 individuals). We showed that brain areas recruited by prosocial behaviors only partially overlap with the mentalizing (dorsal posterior cingulate cortex) and empathy networks (middle cingulate cortex). Additionally, the dorsolateral and ventromedial prefrontal cortices were preferentially activated by prosocial behaviors. Analyses on the functional connectivity profile and functional roles of the neural patterns underlying prosociality revealed that in addition to socio-cognitive and empathic processes, prosocial behaviors further involve evaluation processes and action planning, likely to select the action sequence that best satisfies another person’s needs. By characterizing the multidimensional construct of prosociality at the neural level, we provide insights that may support a better understanding of normal and abnormal social cognition (e.g., psychopathy).
Keywords: prosocial behavior, empathy, mentalizing, activation likelihood estimation, meta-analytic connectivity mapping, resting-state functional connectivity
Introduction
Prosociality refers to behaviors that are intended to benefit others at a cost to the self (de Waal, 2008; Jensen, 2016). Prosocial behaviors comprise a broad range of acts, which include caring, sharing, donating, helping, and volunteering (Dovidio, 2001; Padilla-Walker and Carlo, 2014; Penner et al., 2005). Three aspects are central to prosocial behaviors: (i) an agent intentionally carries out an act (ii) to benefit others (iii) without selfish motivations—as personal gains are an unintended by-product and not the goal of the prosocial act (Batson, 1987; Batson et al., 1981; Jensen, 2016). Previous research has suggested that these behaviors represent a broad and multidimensional construct constituted of cognitive and motivational processes (Coke et al., 1978; Padilla-Walker and Carlo, 2014).
Socio-cognitive abilities (e.g., mentalizing) are required for prosocial acts to understand another person’s needs, infer goals across variable and novel contexts, and recognize when to engage in helping behavior (Warneken, 2015). Humans support those who are in need and ask for help, and they are more likely to do so if the latter are friends or ingroup members (Abrams et al., 2015; Warneken and Tomasello, 2009b; Weller and Hansen Lagattuta, 2013; Young et al., 1999). Prosocial acts require these abilities because they presuppose representations of self- and other-related intentions and goals (Batson, 1991; Brownell et al., 2006; Smiley, 2002), and ontogenetic evidence indicates that the development of complex prosocial behaviors is concomitant with the refinement of mentalizing abilities (Abrams et al., 2015; Eisenberg et al., 2015; Warneken and Tomasello, 2009a).
Further, the motivation to help (e.g., empathic concern) is required to resonate with the other’s needy situation and to be motivated to see the other’s needs satisfied (Dovidio and Penner, 2007). Such motivation has been proposed to be an evolutionary outcome of empathy (de Waal, 2008; Rumble et al., 2009; Xu et al., 2018). Humans exhibit empathic concerns about the welfare of others (Batson, 2011) and based on these concerns, they feel committed to alleviate others’ distress and pain (de Waal, 2008; Warneken, 2015; Warneken and Tomasello, 2009a).
Building on this evidence, a qualitative review of the neuroimaging literature has proposed a set of brain regions that are likely involved in prosociality (Chakroff and Young, 2014). On the one hand, socio-cognitive abilities are instantiated in brain areas broadly associated with the mentalizing network (Bzdok et al., 2012) to support processes that lead to the recognition of another person’s needs and the circumstances in which prosocial behaviors are appropriate. Candidate regions involve brain areas previously implicated in social trait attributions and social event knowledge, such as the posterior cingulate cortex (PCC) and medial prefrontal cortex (mPFC) (Hackel et al., 2015; Krueger et al., 2009; Mitchell et al., 2009; Murray et al., 2012). On the other hand, the motivation for a prosocial act is instantiated in brain regions broadly associated with the empathy network (Bzdok et al., 2012; Fan et al., 2011) to support processes that lead to identifying oneself with another person’s needy situation and thus to the emergence of empathic concerns (Chakroff and Young, 2014). Brain regions like the middle cingulate cortex (MCC) and anterior insula (AI) might play a central role given their involvement in vicarious affective experiences (e.g., pain, disgust, and distress) that promote empathic concern (Corradi-Dell'Acqua et al., 2016; Fan et al., 2011).
One limitation of the current literature is that brain regions involved in prosociality have been inferred from task-based functional MRI (fMRI) investigations of single prosocial behaviors (Hackel et al., 2015; Karns et al., 2017; Moll et al., 2006; Park et al., 2017). Evidence of the common neural network underlying all these prosocial acts is still lacking. Further, small sample sizes and analytic flexibility (combined with publication bias) pertain to other concerns for individual fMRI studies (Feredoes and Postle, 2007; Raemaekers et al., 2007). Moreover, even though a recent comparative meta-analysis has provided preliminary evidence for the involvement of the mPFC in giving behaviors (Cutler and Campbell-Meiklejohn, 2019), it is still unclear to which extent the neural underpinnings of other prosocial behaviors are part of the mentalizing and empathy brain networks hypothesized to be involved in those very behaviors. Because prosociality is such a composite and multidimensional construct, a quantitative meta-analysis of the neuroimaging evidence on its components might be the most suitable means to unearth its neural underpinnings.
Here, we aimed at testing the presence of the above-mentioned cognitive and motivational modules of prosocial behaviors at the neural level and whether there are brain patterns preferentially associated with prosociality. As prosocial behaviors imply carrying out actions that help others, brain regions associated with action-outcome evaluations and action planning might be involved as well. We performed a quantitative meta-analysis implementing the activation likelihood estimation (ALE) algorithm (Eickhoff et al., 2009) and meta-analytic connectivity analyses to identify the meta-analytic neural profile of prosociality, mentalizing, and empathy, and examined their convergences and divergences. Finally, to characterize the functions of the emerging neural patterns, we employed functional decoding (FD) analyses (Eickhoff et al., 2017).
Methods
ALE meta-analyses
Literature search and selection.
In this work, we performed three meta-analyses to identify meta-analytic brain regions consistently engaged by (1) prosociality, (2) mentalizing, and (3) empathy. A systematic online database search was performed on PubMed and Google Scholar by entering various combinations of relevant search items referring to behaviors that the literature had identified as prosocial behaviors. The following keywords were used for the meta-analysis on prosociality: ‘generosity’, ‘altruism’, ‘cooperation’, ‘charity’, ‘donation’, ‘prisoner’s dilemma’, ‘prosocial’, ‘prosociality’, ‘altruistic behavior’, ‘prosocial behavior’, ‘dictator game’, ‘fMRI’, ‘magnetic resonance imaging’, and ‘neuroimaging’, ‘PET’, ‘positron emission tomography’.
The meta-analyses on empathy and mentalizing were built on previous meta-analyses (Bzdok et al., 2012; Fan et al., 2011; Lamm et al., 2011). Given the massive number of new studies on empathy and mentalizing since those meta-analyses, we decided to update this previous work and test whether those results still hold with the new investigations added to the literature. Hence, our work directly builds on those previous meta-analyses (by including the experiments they analyzed) and aims to replicate and extend their results with a larger dataset including the newer studies from the literature, which provides more robust results. Keywords for empathy were as follows: ‘empathy’, ‘empathic’, ‘fMRI’, ‘magnetic resonance imaging’, and ‘neuroimaging’, ‘PET’, ‘positron emission tomography’. Keywords for mentalizing were as follows: ‘mentalizing’, ‘theory of mind’, ‘perspective taking’, ‘false belief’, ‘fMRI’, ‘magnetic resonance imaging’, and ‘neuroimaging’, ‘PET’, ‘positron emission tomography’.
Also, we explored several other sources for all meta-analyses, including (a) the BrainMap database (http://brainmap.org), (b) work cited in review papers, and (c) direct searches on the names of frequently occurring authors. The studies were considered for the meta-analysis if they met the following criteria: (i) participants were free from psychiatric or neurological diagnoses; (ii) participants were adults; (iii) no pharmacological modulations were reported; (iv) fMRI was used as the imaging modality (no PET studies were found under the searched terms); (v) participants underwent a task in which they made a prosocial decision (only for prosociality); (vi) the prosocial decision implied some costs and was not exclusively advantageous to the participants (only for prosociality); (vii) whole-brain analyses were applied (excluding region of interest [ROI] analyses); (viii) fMRI results were derived from a general linear model based on either a binary contrast or parametric analyses in within-subject comparisons; and (ix) activations were presented in a standardized stereotaxic space (Talairach or Montreal Neurological Institute, MNI). Note that Talairach coordinates were converted into MNI space using the GingerALE software (https://www.brainmap.org/ale/) with Brett’s algorithm.
ALE algorithm.
To determine the brain regions consistently activated across the identified studies, we employed a coordinate-based meta-analytic approach using the ALE algorithm (in-house MATLAB 2016b scripts) (Eickhoff et al., 2009). ALE determines the convergence of foci reported from different functional neuroimaging studies with coordinates of peak activations in standardized space (Laird et al., 2005; Turkeltaub et al., 2002). Foci are interpreted as spatial probability distributions. Their widths are based on empirical estimates of the spatial uncertainty based on between-subject and between-template variability of the neuroimaging data (Eickhoff et al., 2009). The sample sizes of the studies reporting the foci are used to weight the between-subject variability. Thereby, the ALE algorithm presupposes a more reliable approximation to the ‘true’ activation for larger sample sizes, which are modeled with smaller Gaussian distributions (Eickhoff et al., 2009).
An ALE map is obtained by calculating the union of the individual modulated activation maps created from the maximum probability associated with anyone's focus (always the closest one) for each voxel (Turkeltaub et al., 2012). This ALE map is determined against a null-distribution of random spatial association between studies employing a nonlinear histogram integration algorithm (Eickhoff et al., 2012; Turkeltaub et al., 2012). Significant results were assessed at a cluster-level family-wise error (cFWE) correction at p < 0.05 with a cluster defining threshold of p < 0.001 and 10,000 permutations (Eickhoff et al., 2012; Eklund et al., 2016). To meet the criteria of robust unbiased results, clusters were considered significant only if the most dominant experiment contributed to the significant cluster on average less than 50%, and the two most dominant experiments contributed, on average less than 80% (Bellucci et al., 2017; Bellucci et al., 2018; Eickhoff et al., 2016). Experimental contributions were represented by the fraction of the ALE value accounted for by each experiment contributing to the significant cluster. This average non-linear contribution to the ALE value was computed from the ratio of the ALE values at the location of the cluster with and without each contributing experiment (Eickhoff et al., 2016).
The following number of experiments, foci, and subjects were found for our three meta-analyses: prosociality had 67 experiments, 433 foci, 1,642 subjects (i.e., an average of 24.7 subjects per experiment; see Supplementary list of study for prosociality); empathy had 273 experiments, 3,267 foci, 5,485 subjects (an average of 21.3 subjects per experiment; see Supplementary list of study for empathy); and mentalizing had 240 experiments, 2,483 foci, 4,867 subjects (an average of 21.3 subjects per experiment; see Supplementary list of study for mentalizing).
Contrast and conjunction of ALE results.
To investigate differences and convergences between the meta-analytic results for prosociality, empathy and mentalizing, two contrast and conjunction analyses were performed. Contrast analyses were based on voxelwise differences of the Z-scores obtained from the ALE maps. Significance was tested with a permutation test. Thereby, the experiments of each meta-analysis were pooled and randomly divided into two groups of the same size as the two original datasets. The ALE scores of these two randomly generated groups were voxelwise subtracted from each other to generate a map of ALE score differences. This procedure was repeated 10,000 times, yielding an empirical null-distribution of maps of ALE score differences. Finally, the ALE maps of ‘true’ differences were thresholded at a posterior probability of p > 95%. Conjunction analyses were performed by computing a minimum conjunction with a cFWE < 0.05 and a voxelwise, cluster-forming threshold of p < 0.001 (Nichols et al., 2005).
Meta-analytic functional profiles
Task-free resting-state functional connectivity analysis.
Task-free resting-state functional connectivity (RSFC) of the prosociality brain regions was estimated from fMRI images of 192 healthy volunteers from the Enhanced Nathan Kline Institute – Rockland Sample, who underwent a resting-state scan right before which they were instructed to look at a fixation cross, not to think of anything in particular and not to fall asleep (Nooner et al., 2012). The reanalysis of the data was approved by the local ethics committee of the Heinrich-Heine University in Düsseldorf. Functional images were acquired with a Siemens TimTrio 3T scanner using a gradient-echo, echo-planar imaging (EPI) pulse sequence (repetition time = 1.4s; echo time = 30 ms, flip angle = 65, voxel size = 2.0 × 2.0 × 2.0 mm; number of slices = 64).
After minimal preprocessing (unwrap and realign), FIX (FMRIB's ICA-based Xnoiseifier, version 1.061 as implemented in FSL 5.0.9) (Griffanti et al., 2014; Salimi-Khorshidi et al., 2014) was used to remove physiological and movement artifacts by decomposing the data into independent components and identifying noise components with the help of a large number of distinct spatial and temporal features via pattern classification. Unique variance related to the artefactual independent components was regressed from the data together with 24 movement parameters (including derivatives and second-order effects as previously described and evaluated) (Satterthwaite et al., 2013). After having excluded the first four scans, functional images were preprocessed using SPM8 (Wellcome Trust Centre for Neuroimaging, London) and in-house MATLAB scripts as follows: functional images were corrected for head movement using a two-pass affine registration (alignment to the initial volume followed by alignment to the mean after the first pass); the mean image was then spatially normalized to the ICBM-152 reference space using the “unified segmentation” approach (Ashburner and Friston, 2005); using the resulting deformation parameters, the individual functional images were subsequently smoothed with a 5-mm full width at half maximum Gaussian kernel to improve the signal-to-noise ratio and compensate for residual anatomic variations. The time-course of each seed was extracted by computing the first eigenvariate of the time-series of all voxels within 5 mm of the seed coordinates.
To reduce spurious correlations, variance explained by the mean white matter and cerebral spinal fluid signal were removed from the timeseries, which was then bandpass filtered with a filter ranging between 0.01 and 0.08 Hz. Afterwards, the functional connectivity profile of each seed was estimated by correlating each seed’s timeseries with the timeseries of all other gray-matter voxels across the brain (Pearson correlation). Correlation coefficients were transformed into Fisher's z-scores, which were entered in a second-level ANOVA for group analysis, including age and sex as covariates of no interest. The data was then subjected to non-parametric permutation-based inference and cluster-level thresholded at p < 0.05 to correct for multiple comparisons.
Task-based meta-analytic connectivity mapping.
The task-based co-activation profile of each prosociality brain region was estimated employing task-based meta-analytic connectivity mapping (MACM), which uses patterns of co-activations reported in standardized space by neuroimaging studies in the BrainMap database (http://www.brainmap.org/) (Laird et al., 2009; Langner et al., 2014) and related to activations of between-condition differences from whole-brain neuroimaging contrasts in healthy individuals. Studies including between-group differences (e.g., age, sex or handedness), effects of interventions (e.g., pharmacological or training-based), and clinical populations were excluded.
We used a 5-mm radius around the peak coordinates of the meta-analytic clusters from the prosociality meta-analysis (i.e., of the four prosociality brain regions). The following experimental contrasts, number of foci and participants were identified for each prosociality brain region: dlPFC (96 experimental contrasts; 1,356 foci; 1,417 participants), dPCC (117 experimental contrasts; 1,415 foci; 1,749 participants), vmPFC (245 experimental contrasts; 2,862 foci; 3854 participants), and MCC (404 experimental contrasts; 5,806 foci; 5,896 participants). Whole-brain peak coordinates of all those studies from BrainMap that reported at least one focus of activation within the respective seeds were downloaded. Coordinates were then analyzed with the ALE algorithm to detect areas of convergence of coactivations with the two seeds. Finally, the ALE maps were thresholded at p < 0.05 cluster-level corrected (voxelwise, cluster-forming threshold: p < 0.001) and converted into z-scores for display.
Conjunction of meta-analytic connectivity profiles.
To determine the robustly interconnected areas from the task-free and task-based meta-analytic connectivity profiles of prosociality, a conjunction analysis was performed using the minimum conjunction of the previous functional connectivity results thresholded with a cFWE < 0.05 and a voxelwise, cluster-forming threshold of p < 0.001 (Nichols et al., 2005). To further single out brain areas that were engaged exclusively by prosociality and neither mentalizing nor empathy, two exclusive conjunctions were conducted between the extended prosociality network and both mentalizing and empathy brain regions identified in the previous ALE analysis. The union of these exclusive maps yielded overlapping brain regions that were preferentially associated with prosociality.
Functional characterization of meta-analytic clusters
The functional role of the prosociality brain regions were characterized based on the behavioral domains (BD)in the BrainMap database by using forward and reverse inference (Müller et al., 2013; Rottschy et al., 2012). In the forward inference, the functional profile was determined by identifying BD for which the probability of finding activation in the respective set of regions was significantly higher than the overall (a priori) chance across the entire database. That is, we tested whether the conditional probability of activation given a particular BD, i.e., P(Activation∣Domain), was higher than the baseline probability of activating the regions in question per se, namely, P(Activation). Significance was established using a binomial test using the standard α = 0.05, corrected for multiple comparisons using the false discovery rate (FDR). In the reverse inference, the functional profile was determined by identifying the most likely BD, given activation in a particular set of regions. This likelihood P(Domain∣Activation) can be derived from P(Activation∣Domain) as well as P(Domain) and P(Activation) using Bayes' rule. Significance was then assessed using a chi-squared test (with FDR < 0.05 for correction of multiple comparisons).
Anatomical labeling and data visualization
The SPM Anatomy toolbox (www.fz-juelich.de/ime/spm_anatomy_toolbox, v.2.2b, Eickhoff et al., 2007), and MRIcron (http://people.cas.sc.edu/rorden/mricron/install.html/) were used for anatomical labeling. MRIcroGL (https://www.mccauslandcenter.sc.edu/mricrogl/home/) was used for brain visualizations (with default settings).
Results
Meta-analytic clusters for prosociality, empathy and mentalizing
We first identified brain regions consistently activated by prosociality, empathy, and mentalizing. For prosociality, we searched both broad and general keywords, such as prosocial and prosocial behaviors, as well as more specific key terms describing single prosocial behaviors, such as generosity, altruism, charity, and donation. Moreover, to be able to include all appropriate and relevant behaviors, scientific paradigms known to experimentally investigate forms of prosocial behaviors were searched as well, like prisoner’s dilemma and dictator game. For empathy and mentalizing, we searched key terms related to these psychological phenomena, such as empathic and theory of mind, similarly to previous work (Bzdok et al., 2012; Fan et al., 2011; Lamm et al., 2011).
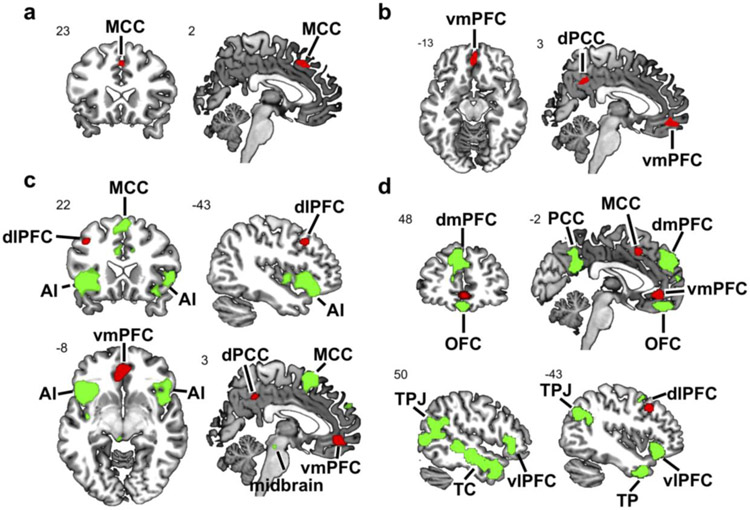
We observed that prosocial behaviors consistently activated the ventromedial PFC (vmPFC), left dorsolateral PFC (dlPFC), MCC, and dorsal PCC (dPCC)—a cluster between the PCC and precuneus (Fig. 1 & Tab. 1 & Tab S1). Replicating and extending previous findings, we observed that empathy consistently activated, among other regions, the bilateral AI, bilateral amygdala, and MCC (Fig. 2a & Tab. S2). Finally, we observed that mentalizing consistently activated, among other regions, the bilateral temporal cortex (TC) extending from the temporoparietal junction (TPJ) to the temporal pole (TP), PCC and mPFC (Fig. 2b & Tab. S3).
Figure 1. Prosociality brain regions.
ALE meta-analysis results showing brain regions consistently engaged by prosocial behaviors. Results were cluster-level familywise-error corrected for multiple comparisons (cFWE < .05). Depicted are Z values. dlPFC, dorsolateral prefrontal cortex; dPCC, dorsal posterior cingulate cortex; MCC, middle cingulate cortex; vmPFC, ventromedial prefrontal cortex; ALE, activation likelihood estimation.
Table 1. Coordinates of prosociality brain regions.
Meta-analytic results for brain regions associated with prosociality. Listed are clusters consistently activated for prosocial behaviors with MNI coordinates (in mm), Z scores at the peak, cluster size (in voxels), number of contributing experiments (with their percentage in parentheses based on the total number of experiments), and the contribution percentages of the most dominant experiments. vmPFC, ventrolateral prefrontal cortex; dlPFC, dorsolateral prefrontal cortex; MCC, middle cingulate cortex; dPCC, dorsal posterior cingulate cortex; BA, Brodmann area; anatomical assignment based on the Anatomy toolbox in parentheses; L, left; ALE, activation likelihood estimation; MNI, Montreal Neurological Institute; MDE, most dominant experiment; 2MDE, two most dominant experiments.
| Brain Regions |
Anatomical location | BA | Coordinates |
Z score |
Cluster Size |
Contributing experiments |
MDE | 2MDE | ||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||||
| vmPFC | middle orbital gyrus | 10 (Fp2/s32) | 0 | 46 | −8 | 6.21 | 263 | 16 (23.9%) | 11.2% | 22.4% |
| L dlPFC | middle frontal gyrus | 44 | −40 | 18 | 40 | 4.71 | 90 | 11 (16.4%) | 23.3% | 43.0% |
| MCC | superior medial gyrus | 32 | 0 | 22 | 42 | 3.42 | 108 | 12 (17.9%) | 17.0% | 32.8% |
| dPCC | 31 | 0 | −52 | 36 | 3.51 | 119 | 9 (13.4%) | 20.3% | 39.3% | |
Figure 2. Empathy and mentalizing brain regions.
ALE meta-analyses results showing brain regions consistently engaged by empathy (a) and mentalizing (b). Results were cluster-level familywise-error corrected for multiple comparisons (cFWE < .05). Depicted are Z values. IPL, inferior parietal lobule; dmPFC, dorsomedial prefrontal cortex; MCC, middle cingulate cortex; AI, anterior insula; TPJ, temporoparietal junction; TP, temporal pole; vlPFC, ventrolateral prefrontal cortex; PCC, posterior cingulate cortex; dmPFC, dorsomedial prefrontal cortex; ALE, activation likelihood estimation.
Converging and diverging neural patterns between prosociality, empathy and mentalizing
Once we identified the meta-analytic clusters consistently activated by prosociality, empathy, and mentalizing, we set out to test to which degree the neural patterns of prosociality include brain regions that are part of the empathy and mentalizing meta-analytic maps. Given the hypothesis on the cognitive level that prosocial behaviors require socio-cognitive and empathic abilities, we should observe a certain degree of convergence. We ran two conjunction analyses between the meta-analytic map for prosociality and those for empathy and mentalizing identified in the previous analyses to estimate these neural convergences (Tab. S4). We observed that the MCC was commonly activated by prosociality and empathy (Fig. 3a), whereas the vmPFC and dPCC were commonly activated by prosociality and mentalizing (Fig. 3b). These results confirm on the neural level that some brain regions associated with empathic and socio-cognitive processes were engaged by prosocial behaviors as well.
Figure 3. Contrast and conjunction analyses.
Conjunction analyses to identify brain regions commonly engaged by prosociality and empathy (a) and by prosociality and mentalizing (b). Contrast analyses to identify diverging neural patterns between prosociality (red) and empathy (green) (c) and between prosociality (red) and mentalizing (green) (d). MCC, middle cingulate cortex; vmPFC, ventromedial prefrontal cortex; dPCC, dorsal posterior cingulate cortex; dlPFC, dorsolateral prefrontal cortex; AI, anterior insula; dmPFC, dorsomedial prefrontal cortex; PCC, posterior cingulate cortex; OFC, orbitofrontal cortex; TPJ, posterior temporoparietal junction; TC, temporal cortex; vlPFC, ventrolateral prefrontal cortex; TP, temporal pole.
However, it is still an open question whether any of the meta-analytic clusters identified by the ALE analyses are preferentially activated by prosocial behaviors but neither empathy nor mentalizing. Therefore, we ran two contrast analyses between the meta-analytic map for prosociality and those for empathy and mentalizing. We observed that the left dlPFC, dPCC, and vmPFC were preferentially activated by prosocial behaviors as opposed to empathy (Fig. 3c & Tab. S5), whereas the MCC, AI, amygdala and inferior parietal lobule (IPL) were preferentially engaged by empathy as opposed to prosociality (Fig. 3c & Tab. S5). On the contrary, in the second contrast analysis between prosociality and mentalizing, we observed that the vmPFC, left dlPFC, and MCC were preferentially activated by prosocial behaviors (Fig. 3d & Tab. S5), while the TPJ, lateral PFC, and dorsal mPFC were preferentially engaged by mentalizing (Fig. 3d & Tab. S5).
These findings suggest that prosocial behaviors involve both socio-cognitive and empathic processes, recruiting some of the brain regions that are part of the mentalizing network (e.g., dPCC) and some of the brain regions that are part of the empathy network (e.g., MCC). However, our contrast analyses further revealed brain regions like the vmPFC and dlPFC that were more consistently engaged by prosocial behaviors than empathy and mentalizing, suggesting a preferential role for these brain regions in prosociality.
Connectivity and co-activation analyses of the prosociality brain regions
We next set out to examine whether the brain regions underlying prosocial behaviors form an interrelated functional connectivity network. A good match between resting-state networks and task networks has been shown before, which has led to the hypothesis that functional networks interact with each other at rest with the same functional hierarchy that is observed during task performance (Cole et al., 2014; Smith et al., 2009). Specifically, resting-state functional connectivity maps onto a layout of activity patterns elicited by different cognitive domains (Raichle, 2015; Tavor et al., 2016). Hence, identifying the meta-analytic functional connectivity networks of a specific brain region during both rest and task, and analyzing their convergence help to robustly determine the consistent functional connectivity profile of that region. We employed two whole-brain connectivity analyses, namely, task-free RSFC and task-based MACM across more than 70 cognitive domains, to first determine the whole-brain functional connectivity layout of each prosociality brain region and then their exclusive convergence for prosociality as opposed to mentalizing or empathy.
These analyses revealed a broad and converging functional connectivity profile for the prosociality brain regions (Fig. 4a-b & Tab. S6-7). In particular, the dPCC and vmPFC were similarly coupled to brain areas such as the TPJ, angular gyrus, and TC, whereas the MCC and dlPFC were more closely coupled with lateral parietal and prefrontal areas like the bilateral IPL and lateral PFC. Further, the vmPFC reveled functional connectivity with the striatum and orbitofrontal cortex, whereas the dlPFC was also functionally coupled with the PCC and TPJ. A conjunction of these connectivity profiles revealed a common functional connectivity network involving the four ALE meta-analytic brain regions (i.e., dlPFC, dPCC, MCC and vmPFC) with functional connectivity with cortical and subcortical regions, like the striatum, bilateral TC and IPL with overlaps in the TPJ (Fig. 4c & Tab. S8).
Figure 4. Functional connectivity and co-activation analyses.
Results of task-free resting-state connectivity (a) and task-based co-activations (b) of the four meta-analytic clusters consistently engaged by prosocial behaviors. Conjunction analyses of the task-free and task-based functional connectivity profiles to identify the extended prosociality network (c). Common brain regions (yellow) of the two maps yielded by the exclusive conjunctions of the prosociality network and empathy (red), and the prosociality network and mentalizing (green) (d). dPCC, dorsal posterior cingulate cortex; dlPFC; dorsolateral prefrontal cortex; vmPFC, ventromedial prefrontal cortex; MCC, middle cingulate cortex; IPL, inferior parietal lobule; TC, temporal cortex; mPFC, medial prefrontal cortex; TPJ, temporoparietal junction; AI, anterior insula; p, prosociality network; e, empathy; m, mentalizing; ∧, logical ‘and’; ¬, logical negation; ∩, conjunction.
Part of these common functional connectivity patterns includes regions previously observed to be associated with the mentalizing and empathy meta-analytic maps. Hence, to single out brain regions preferentially coupled with the meta-analytic activation patterns of prosocial behaviors, we further computed an exclusive conjunction analysis between this functional connectivity profile of prosociality and each of the mentalizing and empathy meta-analytic maps (Fig. 4d). We observed that the striatum, bilateral dlPFC, and IPL were preferentially part of the functional connectivity profile of prosocial behaviors.
Functional characterization of the prosociality brain regions
To gain insights into the different computational processes undertaken by the emerged meta-analytic neural patterns for prosociality, we implemented FD analyses using forward and reverse inferences, which characterized the functional roles of the identified prosociality brain regions. Functional roles were characterized using the BD in the BrainMap database, which describe categories (i.e., action, cognition, emotion, interception, and perception) and subcategories (e.g., reward, social cognition, language, working memory) of the mental operations likely isolated by the experimental contrasts stored in the database (Fox and Lancaster, 2002; Fox et al., 2014; Laird et al., 2009). We performed forward and reverse inferences to identify the experiments that engage a particular brain region, and then analyzed the experimental meta-data that describe the experimental settings employed in the studies (Bellucci et al., 2020; Camilleri et al., 2018)—enabling statistical inference on the type of tasks that evoke activations in a certain brain region.
Forward and reverse inferences provide converging results (Fig. 5a-b). In particular, we found that the dPCC was mainly associated with social cognition and the vmPFC with an affective domain involving fear, reward, and gustative perception, but also with cognitive functions such as reasoning and, like the dPCC but to a lesser extent, social cognition. Both the dlPFC and the MCC were associated with forms of cognitive control, but while the MCC was mainly related to a cognitive domain involving reasoning and semantics, the dlPFC, like the vmPFC, further yielded functional associations with an affective domain. However, the specific affective processes associated with the dlPFC were different from the ones associated with the vmPFC, suggesting different functional roles of these two brain regions in affective processing. These results indicate both similarities and differences in the functional roles of the prosociality brain regions. In particular, they refer to partly converging, but still distinct functional modules undertaking separate aspects of the processes underlying a prosocial act.
Figure 5. Functional decoding analyses.
Forward (a) and reverse (b) functional decoding analyses to characterize the functional roles of the four meta-analytic clusters consistently engaged by prosocial behaviors. For forward functional decoding analyses, the likelihood ratios are depicted, whereas probability values are depicted for reverse functional decoding. dPCC, dorsal posterior cingulate cortex; dlPFC; dorsolateral prefrontal cortex; vmPFC, ventromedial prefrontal cortex; MCC, middle cingulate cortex.
Discussion
Prosocial behaviors refer to a wide range of acts aimed at benefitting others (e.g., helping, sharing, and caring). Psychological accounts posit that the psychological components of prosocial behaviors comprehend socio-cognitive abilities to understand the other’s goals, and empathic concern to be motivated to help. This hypothesis predicts the engagement of the mentalizing and empathy brain networks on the neural level. Here, we found partial evidence to support this hypothesis. In particular, brain regions consistently activated by prosocial behaviors do engage areas associated with mentalizing (dPCC) and empathy (MCC). However, we also observed other brain regions (dlPFC and vmPFC) preferentially activated by prosociality with functional connectivity with subcortical and lateral frontoparietal areas.
Understanding another person’s goals
To act prosocially, an agent has to understand the intentions and goals of another individual. Mentalizing is hence a prerequisite of prosocial behaviors. Socio-cognitive abilities enable the helping agent to realize whether and how particular actions help another to reach her goals (Frith and Frith, 2003; Tomasello et al., 2005). Ontogenetic evidence indicates that only rudimental forms of prosociality are present in preschool children with non-fully developed mentalizing abilities and only in contexts that do not require inferences on complex goals (Brownell et al., 2006; Warneken and Tomasello, 2009a).
Our results showed that prosociality consistently activated the dPCC, which was commonly recruited by prosociality and mentalizing, and was more strongly associated with prosociality than empathy. Connectivity analyses revealed that the dPCC was coupled to brain areas typically part of the mentalizing network (Buckner et al., 2008; Frith and Frith, 2003; Ingvar, 1974) and functional decoding pointed to a role in social cognition for this region. Given these results and previous evidence on its importance in perspective-taking (Cavanna and Trimble, 2006), the dPCC is a good candidate for a role in evaluations of another person’s goals in prosocial behaviors. A previous fMRI study found the dPCC to be recruited for intentional causality and prospective memory in a task where participants had to identify the intentions of consequential actions and bear in mind an intention to act (den Ouden et al., 2005). With this respect, the dPCC might help an agent to prospectively embed her prosocial act into an action sequence that conforms to another individual’s goals. This hypothesis is also consistent with other functions attributed to the dPCC, such as visual imagery in episodic memory (Fletcher et al., 1995; Fletcher et al., 1996) and preparation and execution of intentionally guided actions (Astafiev et al., 2003).
Contrary to the predictions of a previous qualitative review of the neural correlates of prosociality (Chakroff and Young, 2014), we did not observe any consistent activations in the TPJ. Unlike the dPCC, the TPJ has been associated with belief representations and with the justification of a belief (Koster-Hale et al., 2017; Young et al., 2010). Interestingly, the neural signal in the TPJ is predictive only of prosocial behaviors in situations of advantageous inequality (e.g., giving to those who are poorer but not wealthier than oneself) with no links to other prosocial behaviors (Morishima et al., 2012). Even though the TPJ was not preferentially recruited by prosocial behaviors in our study, it was still part of the functional connectivity network of the prosociality brain regions, suggesting that it plays an essential role in supporting their underlying computations. Future work might consider clarifying the role of intentionality and perspective-taking in prosocial behaviors to provide better insights into the involvement of specific mentalizing brain regions.
Motivated to help others
Further, prosocial behaviors require a motivation to help another—arguably triggered by empathic concerns (Batson et al., 1987; Coke et al., 1978; Eisenberg et al., 1994). Individual differences in empathic skills (Bird and Viding, 2014; Blair, 2005; Decety and Jackson, 2004) have been linked to prosocial tendencies and prosocial learning, that is, learning when to benefit others (Lockwood et al., 2016; Lockwood et al., 2014). Importantly, vicarious distress is not enough to motivate prosocial behaviors if it is not accompanied by other-oriented empathic concerns (FeldmanHall et al., 2015).
In our study, the only brain area consistently engaged by prosocial behaviors that overlapped with the empathy network was the MCC. Connectivity analyses revealed that this region was functionally coupled with lateral parietal and prefrontal areas generally part of a central-executive network (Bressler and Menon, 2010). Functional decoding analyses characterize the MCC as having a specific role in cognitive control. These results are in line with previous studies proposing cognitive-motor control as the core functional computation of this region (Botvinick et al., 2004; Guo et al., 2013; Hoffstaedter et al., 2014; Shackman et al., 2011; Shenhav et al., 2014; Singer et al., 2004). As our results indicate that the MCC was preferentially related to a cognitive domain rather than an affective or social domain, the MCC might be a good candidate for representations of empathic concern, signaling the motivational component of empathy that prompts to take action to change the emotionally painful situation of another person’s needy condition.
Contrary to previous hypotheses, we found no associations between prosociality and the AI, which is considered a central region to empathic processes (Lamm et al., 2011; Lamm et al., 2017; Singer et al., 2004). These results might reflect the two different roles of the AI and MCC in empathy. Unlike some neuroimaging accounts that lump together the functions of the AI and MCC in a general role in “affect sharing” (Lamm et al., 2017), others suggest that the MCC represents a cognitive-evaluative form of empathy, whereas the AI represents an affective-perceptual form (Fan et al., 2011). Our results seem to confirm this functional distinction, suggesting that the cognitive form of empathy, in particular, its motivational component, might play a more central role in prosociality.
Enacting prosocial behaviors
Further, our contrast analyses demonstrated that the vmPFC and, in particular, the dlPFC were preferentially associated with prosociality as opposed to empathy and mentalizing. Our functional decoding analyses revealed that these regions were closely related to both cognitive and affective domains, suggesting that they integrate cognitive and affective signals relevant for prosocial behaviors.
Functional connectivity analyses revealed functional coupling of the vmPFC with both mentalizing areas (e.g., TPJ) and subcortical brain regions (e.g., striatum). Functional decoding analyses suggested a role for the vmPFC in both socio-cognitive and affective processes. Given these results and previous evidence on its role in signaling outcome expectancies and behaviorally-relevant value information (Bellucci et al., 2019a; Bellucci et al., 2019b; Iigaya et al., 2019; Na et al., 2019), the vmPFC might be responsible for the integration of cognitive and affective signals in prosocial behaviors to prospectively evaluate actions and outcomes associated with a prosocial act. In particular, in concert with the striatum and TPJ, which have been shown to play a role in feelings of happiness during generous decisions (Park et al., 2017), the vmPFC might evaluate the positive consequences that a prosocial act has for the helping agent. This resonates with a recent study indicating that individuals report the positive feelings following a prosocial act to be the second-most influential reason to behave prosocially (after "helping those in need" and before "feelings of empathy") (Sisco and Weber, 2019).
Importantly, the dlPFC was the only brain area preferentially engaged by prosocial behaviors and activated by neither empathy nor mentalizing. Connectivity analyses linked the dlPFC with both cognitive control regions (e.g., parietal cortex) and mentalizing regions (e.g., PCC, TPJ). Functional decoding analyses linked the dlPFC to roles in both cognitive and affective processing. This configuration of activation patterns involving the dlPFC, PCC and parietal cortex is often observed when flexible behavioral adaptation to the current social interaction is required based on beliefs about the character and reputation of the interacting partner (Hackel et al., 2015; Igelström et al., 2016; Mende-Siedlecki et al., 2013). A recent neuroimaging study has demonstrated that neural signal in these regions is not only informative of the character traits of another person but also predicts an individual’s willingness to trust that person in a later interaction (Bellucci et al., 2019a). Hence, our results suggest that the recruitment of the dlPFC allows the tracking of another person’s behavior for inferences on her intentions and planning on the adequate course of actions.
Limitations
A couple of limitations have to be addressed. First, the neuroimaging literature, unlike psychological work, has not yet tackled the specific neural patterns underlying the different components of empathy (with the exception of some attempts (Fan et al., 2011)), leaving in particular the neural signatures of empathic concerns underspecified. Given its role in cognitive-motor control, we have here proposed that the MCC might signal behaviorally-relevant motivational states, such as empathic concern (Kolling et al., 2016). However, as neuroimaging studies have so far primarily neglected this motivational component of empathy and the BrainMap does not even contain a behavioral domain for motivation or empathic concern in its catalog, we were here unable to test this hypothesis. Second, we here aimed at identifying the common neural patterns underlying prosocial acts. However, it still remains an open question whether different types of prosocial acts recruit differing neural patterns. Given the few experiments for each of the prosocial acts here considered, future studies are needed to tackle this question. Moreover, we note that most investigations on prosociality use economic paradigms (see Supplementary list of studies for prosociality). For more robust and generalizable results, future studies might consider other types of paradigms, such as vignettes to test individuals’ tendency to prosocial behaviors in different contexts (like in studies on third-party punishment; Bellucci et al., 2020), virtual reality to more ecologically simulate situations requiring prosocial actions or longitudinal studies with chronic (as opposed to acute) manipulation of prosocial behaviors to investigate the effects of being prosocial on the brain (Lyubomirsky et al., 2005; Park et al., 2017). For these future studies, our results provide meta-analytic clusters that could be used as regions of interest to test specific hypotheses in investigations with other methods such as functional near-infrared spectroscopy and transcranial magnetic stimulation. Despite these limitations, we were able to reliably characterize the specific activation patterns, connectivity profile and functional roles of brain areas consistently activated by prosocial behaviors.
Conclusions
Taken together, we found a set of brain regions that were consistently activated by prosocial behaviors. These activation patterns partially overlapped with mentalizing and empathy brain regions, lending support to the hypothesis based on psychological research that socio-cognitive and empathic abilities are central to prosociality. However, we also found that the vmPFC and, in particular, the dlPFC were preferentially recruited by prosocial acts, suggesting that prosocial behaviors require the involvement of other important processes. Analyses on their functional connectivity profile and functional roles suggest that the vmPFC and dlPFC might be involved in valuation and planning of prosocial actions, respectively. These results clarify the role of mentalizing and empathic abilities in prosociality and provides useful insights into the neuropsychological processes underlying human social behaviors. For instance, they might help understand where and how things go awry in different neural and behavioral disorders such as psychopathy and antisocial behavior (Blair, 2007).
Supplementary Material
Highlights.
A psychological proposal posits prosociality engages brain regions of the mentalizing and empathy networks
Our meta-analysis provides only partial support to this proposal.
Prosocial behaviors engage brain regions associated with socio-cognitive and empathic abilities
However, they also engage brain regions associated with evaluation and planning.
Acknowledgments
G.B. is supported by the Max Planck Society. S.B.E. is supported by the National Institute of Mental Health (R01-MH074457), the Helmholtz Portfolio Theme "Supercomputing and Modeling for the Human Brain" and the European Union’s Horizon 2020 Research and Innovation Programme under Grant Agreement No. 785907 (HBP SGA2). We finally would like to acknowledge the funding that has supported the enhanced NKI-RS project, from which the resting-state data were obtained (NIMH BRAINS R01MH094639-01).
Footnotes
Conflict of Interest
The authors declare no conflicts of interest, financial or otherwise.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams D, Van de Vyver J, Pelletier J, Cameron L, 2015. Children's prosocial behavioural intentions towards outgroup members. Br J Dev Psychol 33, 277–294. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ, 2005. Unified segmentation. Neuroimage 26, 839–851. [DOI] [PubMed] [Google Scholar]
- Astafiev SV, Shulman GL, Stanley CM, Snyder AZ, Van Essen DC, Corbetta M, 2003. Functional Organization of Human Intraparietal and Frontal Cortex for Attending, Looking, and Pointing. The Journal of Neuroscience 23, 4689–4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batson CD, 1987. Prosocial motivation: is it ever truly altruistic? Advances in Experimental Social Psychology 20. [Google Scholar]
- Batson CD, 1991. The altruism question: Toward a social-psychological answer. Lawrence Erlbaum Associates, Inc., Hillsdale, NJ, US. [Google Scholar]
- Batson CD, 2011. Altruism in Humans. Oxford University Press, Inc., Oxford New York. [Google Scholar]
- Batson CD, Duncan BD, Ackerman P, Buckley T, Birch K, 1981. Is empathic emotion a source of altruistic motivation? Journal of Personality and Social Psychology 40, 290–302. [Google Scholar]
- Batson CD, Fultz J, Schoenrade PA, 1987. Distress and empathy: two qualitatively distinct vicarious emotions with different motivational consequences. J Pers 55, 19–39. [DOI] [PubMed] [Google Scholar]
- Bellucci G, Camilleri JA, Iyengar V, Eickhoff SB, Krueger F, 2020. The Emerging Neuroscience of Social Punishment: Meta-Analytic Evidence. Neuroscience & Biobehavioral Reviews. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellucci G, Chernyak SV, Goodyear K, Eickhoff SB, Krueger F, 2017. Neural signatures of trust in reciprocity: A coordinate-based meta-analysis. Hum Brain Mapp 38, 1233–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellucci G, Feng C, Camilleri J, Eickhoff SB, Krueger F, 2018. The role of the anterior insula in social norm compliance and enforcement: Evidence from coordinate-based and functional connectivity meta-analyses. Neurosci Biobehav Rev 92, 378–389. [DOI] [PubMed] [Google Scholar]
- Bellucci G, Molter F, Park SQ, 2019a. Neural representations of honesty predict future trust behavior. Nat Commun 10, 5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellucci G, Munte TF, Park SQ, 2019b. Resting-state dynamics as a neuromarker of dopamine administration in healthy female adults. J Psychopharmacol 33, 955–964. [DOI] [PubMed] [Google Scholar]
- Bird G, Viding E, 2014. The self to other model of empathy: providing a new framework for understanding empathy impairments in psychopathy, autism, and alexithymia. Neurosci Biobehav Rev 47, 520–532. [DOI] [PubMed] [Google Scholar]
- Blair RJ, 2005. Responding to the emotions of others: dissociating forms of empathy through the study of typical and psychiatric populations. Conscious Cogn 14, 698–718. [DOI] [PubMed] [Google Scholar]
- Blair RJ, 2007. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends Cogn Sci 11, 387–392. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS, 2004. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci 8, 539–546. [DOI] [PubMed] [Google Scholar]
- Bressler SL, Menon V, 2010. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci 14, 277–290. [DOI] [PubMed] [Google Scholar]
- Brownell CA, Ramani GB, Zerwas S, 2006. Becoming a social partner with peers: cooperation and social understanding in one- and two-year-olds. Child Dev 77, 803–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL, 2008. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124, 1–38. [DOI] [PubMed] [Google Scholar]
- Bzdok D, Schilbach L, Vogeley K, Schneider K, Laird AR, Langner R, Eickhoff SB, 2012. Parsing the neural correlates of moral cognition: ALE meta-analysis on morality, theory of mind, and empathy. Brain Structure and Function 217, 783–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri JA, Muller VI, Fox P, Laird AR, Hoffstaedter F, Kalenscher T, Eickhoff SB, 2018. Definition and characterization of an extended multiple-demand network. Neuroimage 165, 138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR, 2006. The precuneus: a review of its functional anatomy and behavioural correlates. Brain : a journal of neurology 129, 564–583. [DOI] [PubMed] [Google Scholar]
- Chakroff A, Young L, 2014. The Prosocial Brain: Perceiving Others in Need and Acting on it, in: Padilla-Walker LM, Carlo G (Eds.), Prosocial Development: A Multidimensional Approach. Oxford University Press, Oxford, pp. 90–111. [Google Scholar]
- Coke JS, Batson CD, McDavis K, 1978. Empathic mediation of helping: A two-stage model. Journal of Personality and Social Psychology 36, 752–766. [Google Scholar]
- Cole MW, Bassett DS, Power JD, Braver TS, Petersen SE, 2014. Intrinsic and task-evoked network architectures of the human brain. Neuron 83, 238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradi-Dell'Acqua C, Tusche A, Vuilleumier P, Singer T, 2016. Cross-modal representations of first-hand and vicarious pain, disgust and fairness in insular and cingulate cortex. Nat Commun 7, 10904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler J, Campbell-Meiklejohn D, 2019. A comparative fMRI meta-analysis of altruistic and strategic decisions to give. Neuroimage 184, 227–241. [DOI] [PubMed] [Google Scholar]
- de Waal FB, 2008. Putting the altruism back into altruism: the evolution of empathy. Annu Rev Psychol 59, 279–300. [DOI] [PubMed] [Google Scholar]
- Decety J, Jackson PL, 2004. The functional architecture of human empathy. Behav Cogn Neurosci Rev 3, 71–100. [DOI] [PubMed] [Google Scholar]
- den Ouden HE, Frith U, Frith C, Blakemore SJ, 2005. Thinking about intentions. Neuroimage 28, 787–796. [DOI] [PubMed] [Google Scholar]
- Dovidio J, 2001. Adulthood: Prosocial Behavior and Empathy, International Encyclopedia of the Social & Behavioral Sciences, pp. 159–162. [Google Scholar]
- Dovidio JF, Penner LA, 2007. Helping and Altruism, in: Fletcher GJO, Clark MS (Eds.), Blackwell Handbook of Social Psychology: Interpersonal Processes, pp. 162–195. [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT, 2012. Activation likelihood estimation meta-analysis revisited. Neuroimage 59, 2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Constable RT, Yeo BT, 2017. Topographic organization of the cerebral cortex and brain cartography. Neuroimage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT, 2009. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Human brain mapping 30, 2907–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Nichols TE, Laird AR, Hoffstaedter F, Amunts K, Fox PT, Bzdok D, Eickhoff CR, 2016. Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. Neuroimage 137, 70–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, Amunts K, 2007. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage 36, 511–521. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA, Murphy B, Karbon M, Maszk P, Smith M, O'Boyle C, Suh K, 1994. The relations of emotionality and regulation to dispositional and situational empathy-related responding. J Pers Soc Psychol 66, 776–797. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Spinrad TL, Knafo-Noam A, 2015. Prosocial Development, in: Lerner RM (Ed.), Handbook of Child Psychology and Developmental Science, pp. 1–47. [Google Scholar]
- Eklund A, Nichols TE, Knutsson H, 2016. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences of the United States of America 113, 7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Duncan NW, de Greck M, Northoff G, 2011. Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neurosci Biobehav Rev 35, 903–911. [DOI] [PubMed] [Google Scholar]
- FeldmanHall O, Dalgleish T, Evans D, Mobbs D, 2015. Empathic concern drives costly altruism. Neuroimage 105, 347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feredoes E, Postle BR, 2007. Localization of load sensitivity of working memory storage: quantitatively and qualitatively discrepant results yielded by single-subject and group-averaged approaches to fMRI group analysis. Neuroimage 35, 881–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Frith CD, Baker SC, Shallice T, Frackowiak RS, Dolan RJ, 1995. The mind's eye--precuneus activation in memory-related imagery. Neuroimage 2, 195–200. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Shallice T, Frith CD, Frackowiak RS, Dolan RJ, 1996. Brain activity during memory retrieval. The influence of imagery and semantic cueing. Brain : a journal of neurology 119 (Pt 5), 1587–1596. [DOI] [PubMed] [Google Scholar]
- Fox PT, Lancaster JL, 2002. Mapping context and content: the BrainMap model. Nature Reviews Neuroscience 3, 319–321. [DOI] [PubMed] [Google Scholar]
- Fox PT, Lancaster JL, Laird AR, Eickhoff SB, 2014. Meta-Analysis in Human Neuroimaging: Computational Modeling of Large-Scale Databases. Annual Review of Neuroscience 37, 409–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U, Frith CD, 2003. Development and neurophysiology of mentalizing. Philosophical transactions of the Royal Society of London. Series B, Biological sciences 358, 459–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffanti L, Salimi-Khorshidi G, Beckmann CF, Auerbach EJ, Douaud G, Sexton CE, Zsoldos E, Ebmeier KP, Filippini N, Mackay CE, Moeller S, Xu J, Yacoub E, Baselli G, Ugurbil K, Miller KL, Smith SM, 2014. ICA-based artefact removal and accelerated fMRI acquisition for improved resting state network imaging. Neuroimage 95, 232–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Zheng L, Zhu L, Li J, Wang Q, Dienes Z, Yang Z, 2013. Increased neural responses to unfairness in a loss context. Neuroimage 77, 246–253. [DOI] [PubMed] [Google Scholar]
- Hackel LM, Doll BB, Amodio DM, 2015. Instrumental learning of traits versus rewards: dissociable neural correlates and effects on choice. Nature neuroscience 18, 1233–1235. [DOI] [PubMed] [Google Scholar]
- Hoffstaedter F, Grefkes C, Caspers S, Roski C, Palomero-Gallagher N, Laird AR, Fox PT, Eickhoff SB, 2014. The role of anterior midcingulate cortex in cognitive motor control: evidence from functional connectivity analyses. Hum Brain Mapp 35, 2741–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igelström KM, Webb TW, Kelly YT, Graziano MS, 2016. Topographical Organization of Attentional, Social, and Memory Processes in the Human Temporoparietal Cortex. eNeuro 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iigaya K, Hauser TU, Kurth-Nelson Z, O’Doherty JP, Dayan P, Dolan RJ, 2019. Hippocampal-midbrain circuit enhances the pleasure of anticipation in the prefrontal cortex. bioRxiv. [Google Scholar]
- Ingvar DH, 1974. Patterns of brain activity revealed by measurements of regional cerebral blood flow, Alfred Benzon Symposium VIII, Copenhagen. [Google Scholar]
- Jensen K, 2016. Prosociality. Curr Biol 26, R748–752. [DOI] [PubMed] [Google Scholar]
- Karns CM, Moore WE 3rd, Mayr U, 2017. The Cultivation of Pure Altruism via Gratitude: A Functional MRI Study of Change with Gratitude Practice. Front Hum Neurosci 11, 599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolling N, Wittmann MK, Behrens TE, Boorman ED, Mars RB, Rushworth MF, 2016. Value, search, persistence and model updating in anterior cingulate cortex. Nat Neurosci 19, 1280–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster-Hale J, Richardson H, Velez N, Asaba M, Young L, Saxe R, 2017. Mentalizing regions represent distributed, continuous, and abstract dimensions of others' beliefs. Neuroimage 161, 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger F, Barbey AK, Grafman J, 2009. The medial prefrontal cortex mediates social event knowledge. Trends Cogn Sci 13, 103–109. [DOI] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Kurth F, Fox PM, Uecker AM, Turner JA, Robinson JL, Lancaster JL, Fox PT, 2009. ALE Meta-Analysis Workflows Via the Brainmap Database: Progress Towards A Probabilistic Functional Brain Atlas. Front Neuroinform 3, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT, 2005. ALE meta-analysis: Controlling the false discovery rate and performing statistical contrasts. Human brain mapping 25, 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T, 2011. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage 54, 2492–2502. [DOI] [PubMed] [Google Scholar]
- Lamm C, Rütgen M, Wagner IC, 2017. Imaging empathy and prosocial emotions. Neurosci Lett. [DOI] [PubMed] [Google Scholar]
- Langner R, Rottschy C, Laird AR, Fox PT, Eickhoff SB, 2014. Meta-analytic connectivity modeling revisited: controlling for activation base rates. NeuroImage 99, 559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood PL, Apps MA, Valton V, Viding E, Roiser JP, 2016. Neurocomputational mechanisms of prosocial learning and links to empathy. Proceedings of the National Academy of Sciences of the United States of America 113, 9763–9768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood PL, Seara-Cardoso A, Viding E, 2014. Emotion regulation moderates the association between empathy and prosocial behavior. PLoS One 9, e96555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubomirsky S, King L, Diener E, 2005. The benefits of frequent positive affect: does happiness lead to success? Psychol Bull 131, 803–855. [DOI] [PubMed] [Google Scholar]
- Mende-Siedlecki P, Cai Y, Todorov A, 2013. The neural dynamics of updating person impressions. Social cognitive and affective neuroscience 8, 623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Ames DL, Jenkins AC, Banaji MR, 2009. Neural correlates of stereotype application. J Cogn Neurosci 21, 594–604. [DOI] [PubMed] [Google Scholar]
- Moll J, Krueger F, Zahn R, Pardini M, de Oliveira-Souza R, Grafman J, 2006. Human fronto-mesolimbic networks guide decisions about charitable donation. Proc Natl Acad Sci U S A 103, 15623–15628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima Y, Schunk D, Bruhin A, Ruff, Christian C, Fehr E, 2012. Linking Brain Structure and Activation in Temporoparietal Junction to Explain the Neurobiology of Human Altruism. Neuron 75, 73–79. [DOI] [PubMed] [Google Scholar]
- Müller VI, Cieslik EC, Laird AR, Fox PT, Eickhoff SB, 2013. Dysregulated left inferior parietal activity in schizophrenia and depression: functional connectivity and characterization. Front Hum Neurosci 7, 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RJ, Schaer M, Debbane M, 2012. Degrees of separation: a quantitative neuroimaging meta-analysis investigating self-specificity and shared neural activation between self- and other-reflection. Neurosci Biobehav Rev 36, 1043–1059. [DOI] [PubMed] [Google Scholar]
- Na S, Chung D, Hula A, Jung J, Fiore VG, Dayan P, Gu X, 2019. Humans use forward thinking to exert social control. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB, 2005. Valid conjunction inference with the minimum statistic. Neuroimage 25, 653–660. [DOI] [PubMed] [Google Scholar]
- Nooner KB, Colcombe SJ, Tobe RH, Mennes M, Benedict MM, Moreno AL, Panek LJ, Brown S, Zavitz ST, Li Q, Sikka S, Gutman D, Bangaru S, Schlachter RT, Kamiel SM, Anwar AR, Hinz CM, Kaplan MS, Rachlin AB, Adelsberg S, Cheung B, Khanuja R, Yan C, Craddock CC, Calhoun V, Courtney W, King M, Wood D, Cox CL, Kelly AM, Di Martino A, Petkova E, Reiss PT, Duan N, Thomsen D, Biswal B, Coffey B, Hoptman MJ, Javitt DC, Pomara N, Sidtis JJ, Koplewicz HS, Castellanos FX, Leventhal BL, Milham MP, 2012. The NKI-Rockland Sample: A Model for Accelerating the Pace of Discovery Science in Psychiatry. Frontiers in neuroscience 6, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla-Walker LM, Carlo G, 2014. The Study of Prosocial Behavior: Past, Present, Future, in: Padilla-Walker LM, Carlo G (Eds.), Prosocial Development: A Multidimensional Approach. Oxford University Press, Oxford, pp. 3–16. [Google Scholar]
- Park SQ, Kahnt T, Dogan A, Strang S, Fehr E, Tobler PN, 2017. A neural link between generosity and happiness. Nat Commun 8, 15964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner LA, Dovidio JF, Piliavin JA, Schroeder DA, 2005. Prosocial Behavior: Multilevel Perspectives. Annual Review of Psychology 56, 365–392. [DOI] [PubMed] [Google Scholar]
- Raemaekers M, Vink M, Zandbelt B, van Wezel RJ, Kahn RS, Ramsey NF, 2007. Test-retest reliability of fMRI activation during prosaccades and antisaccades. Neuroimage 36, 532–542. [DOI] [PubMed] [Google Scholar]
- Raichle ME, 2015. The restless brain: how intrinsic activity organizes brain function. Philosophical transactions of the Royal Society of London. Series B, Biological sciences 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottschy C, Caspers S, Roski C, Reetz K, Dogan I, Schulz JB, Zilles K, Laird AR, Fox PT, Eickhoff SB, 2012. Differentiated parietal connectivity of frontal regions for “what” and “where” memory. Brain Structure and Function 218, 1551–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumble AC, Van Lange PAM, Parks CD, 2009. The benefits of empathy: When empathy may sustain cooperation in social dilemmas. European Journal of Social Psychology, n/a-n/a. [Google Scholar]
- Salimi-Khorshidi G, Douaud G, Beckmann CF, Glasser MF, Griffanti L, Smith SM, 2014. Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. Neuroimage 90, 449–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Eickhoff SB, Hakonarson H, Gur RC, Gur RE, Wolf DH, 2013. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. NeuroImage 64, 240–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ, 2011. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci 12, 154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Straccia MA, Cohen JD, Botvinick MM, 2014. Anterior cingulate engagement in a foraging context reflects choice difficulty, not foraging value. Nat Neurosci 17, 1249–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD, 2004. Empathy for pain involves the affective but not sensory components of pain. Science 303, 1157–1162. [DOI] [PubMed] [Google Scholar]
- Sisco MR, Weber EU, 2019. Examining charitable giving in real-world online donations. Nature Communications 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley PA, 2002. Intention Understanding and Partner-Sensitive Behaviors in Young Children’s Peer Interactions. Social Development 10, 330–354. [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF, 2009. Correspondence of the brain's functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America 106, 13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavor I, Parker Jones O, Mars RB, Smith SM, Behrens TE, Jbabdi S, 2016. Task-free MRI predicts individual differences in brain activity during task performance. Science 352, 216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasello M, Carpenter M, Call J, Behne T, Moll H, 2005. Understanding and sharing intentions: the origins of cultural cognition. Behav Brain Sci 28, 675–691; discussion 691–735. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA, 2002. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage 16, 765–780. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P, 2012. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Human brain mapping 33, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warneken F, 2015. Precocious Prosociality: Why Do Young Children Help? Child Development Perspectives 9, 1–6. [Google Scholar]
- Warneken F, Tomasello M, 2009a. The roots of human altruism. Br J Psychol 100, 455–471. [DOI] [PubMed] [Google Scholar]
- Warneken F, Tomasello M, 2009b. Varieties of altruism in children and chimpanzees. Trends Cogn Sci 13, 397–402. [DOI] [PubMed] [Google Scholar]
- Weller D, Hansen Lagattuta K, 2013. Helping the in-group feels better: children's judgments and emotion attributions in response to prosocial dilemmas. Child Dev 84, 253–268. [DOI] [PubMed] [Google Scholar]
- Xu X, Liu C, Zhou X, Chen Y, Gao Z, Zhou F, Kou J, Becker B, Kendrick K, 2018. Oxytocin facilitates self-serving rather than altruistic tendencies in competitive social interactions via orbitofrontal cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L, Camprodon JA, Hauser M, Pascual-Leone A, Saxe R, 2010. Disruption of the right temporoparietal junction with transcranial magnetic stimulation reduces the role of beliefs in moral judgments. Proc Natl Acad Sci U S A 107, 6753–6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SK, Fox NA, Zahn-Waxler C, 1999. The relations between temperament and empathy in 2-year-olds. Developmental Psychology 35, 1189–1197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.