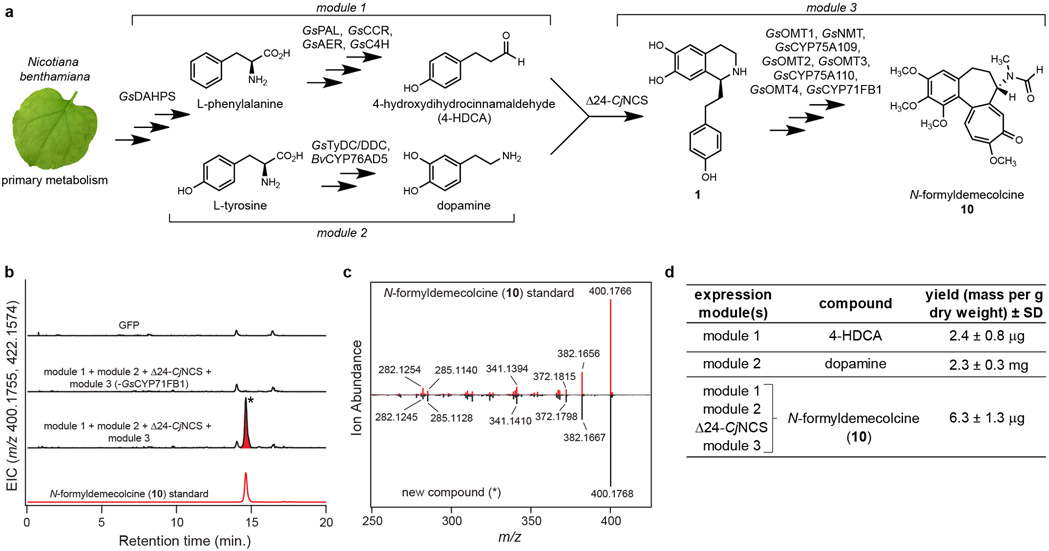
Figure 5. Engineered biosynthesis of N-formyldemecolcine (10) from primary metabolism in Nicotiana benthamiana.
a) Biosynthetic scheme depicting the combination of three biosynthetic modules and CjNCS from benzylisoquinoline alkaloid (BIA) biosynthesis for the engineered production of 10 in N. benthamiana. b) LC-MS chromatograms demonstrating formation of 10 ([M+H]+ = 400.1755 Da, [M+Na]+ = 422.1574 Da) via transient pathway expression in N. benthamiana leaves, as compared to an authentic standard of 10. EIC = extracted ion chromatogram. Metabolic engineering of 10 was repeated >3 times with similar results observed in each experiment. c) MS/MS fragmentation (m/z = 400.1755, 20V) of the new compound highlighted in panel b compared to that of the 10 standard. d) Calculated yield of products synthesized via module 1 (4-HDCA), module 2 (dopamine), and the full engineered pathway (10). Yields are reported as mass of compound per gram dry weight of extracted N. benthamiana leaf tissue (n=3 independent replicates for each experiment), along with the corresponding standard deviation (SD). See Supplementary Information for a discussion of yield comparisons to native colchicine-producing plants.