Figure 3. Tertiary structures of CD36 and SR-B1.
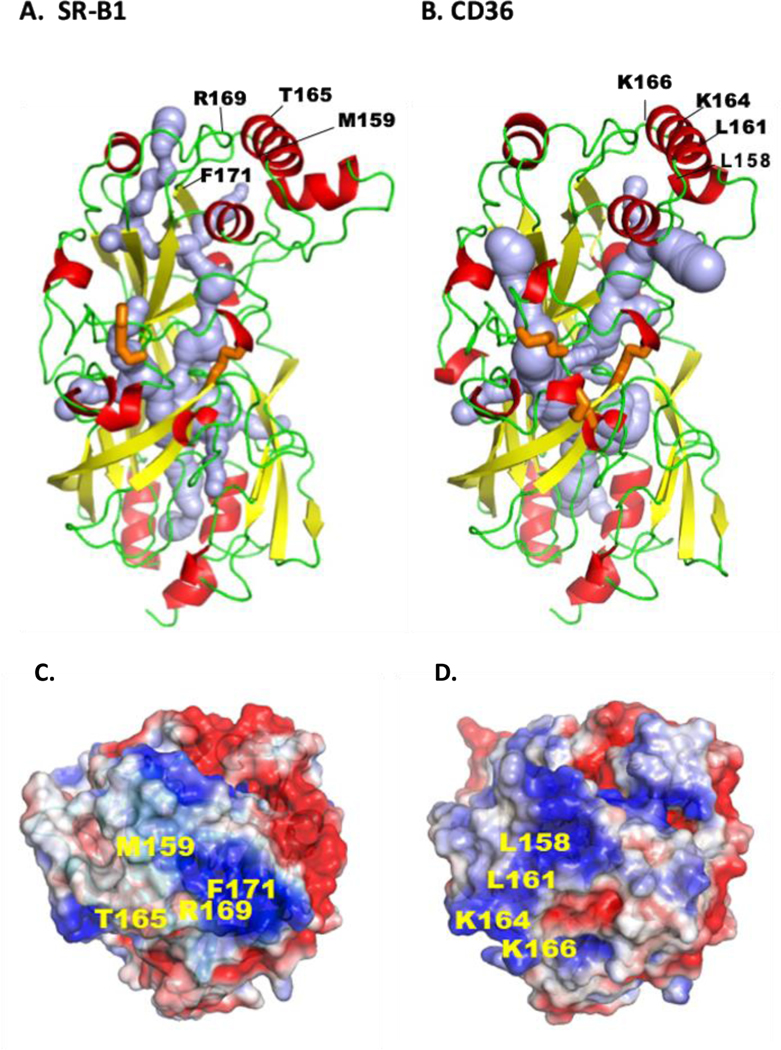
Both receptors are members of the CD36 family that also includes LIMP2, and both play important roles in endothelial lipid transport. The comparison is based on the crystal structures. The ectodomain of human CD36 (A) is derived from its crystallography and homology modeling of human SR-BI (B). For both proteins the α-helices are in red, the β-sheets in yellow and the loops in green. Crystal structure identified a hydrophobic internal lipid transport tunnel (light blue) that traverses the length of these proteins ending at the bilayer proximity. This tunnel can be accessed from the apex region and residues identified to be important for ligand binding are highlighted. C and D show the apex regions’ electrostatic surface potential for SR-B1 (C) and CD36 (D) and residues implicated in binding of LDL and HDL (SR-B1), and FA and oxidized-LDL (CD36) are indicated. The tunnel is surrounded by short α-helices and the disulfide bridges (orange) would stabilize structure and dimensions of the cavity for it to accommodate the lipid ligands. E shows linear sequence of the C-terminal domains for SR-B1 and CD36 highlighting the PDZ binding sequence (red) of SR-B1 and the palmitoylated (green) and ubiquitinated (orange) residues of CD36 important for signaling. The tertiary structures were rendered in PyMol211, cavity prediction used CaverWeb 1.0212 and the electrostatic surface potential map used APBS212 with color gradient of −2 (red) to +2 (blue) kT/e.
