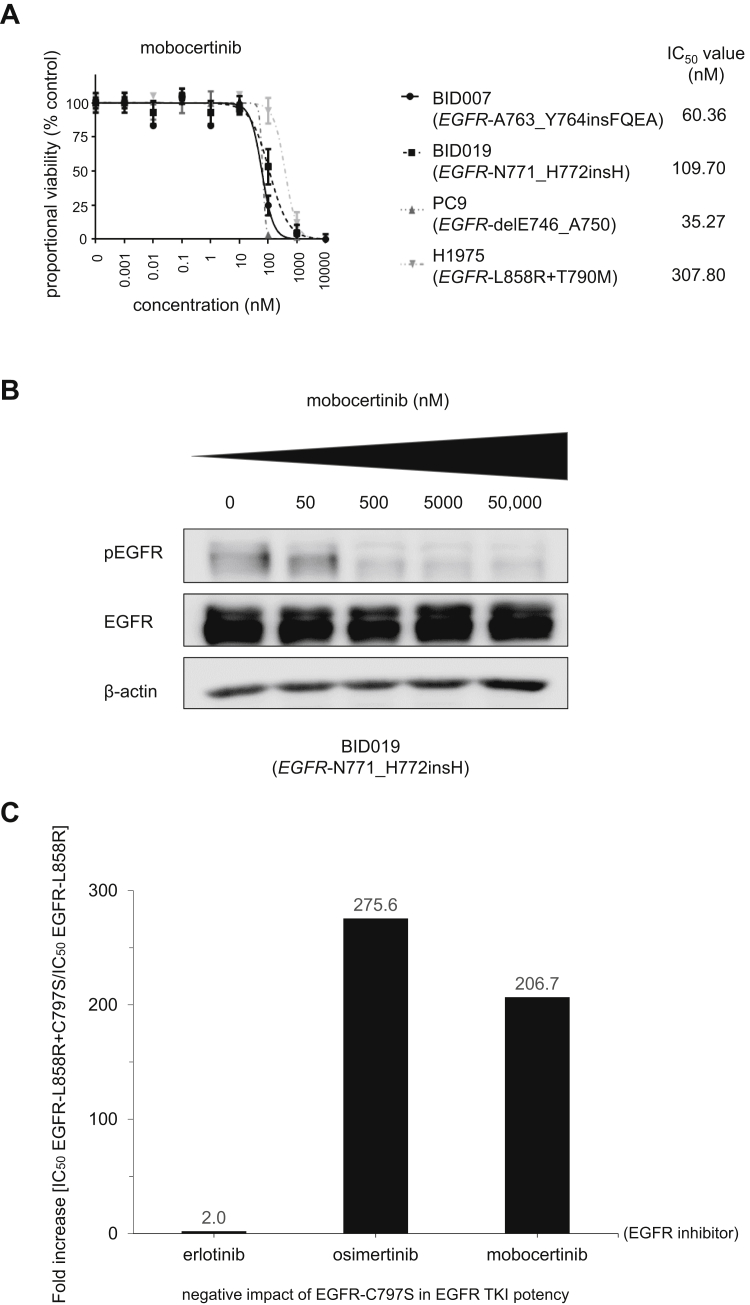
Figure 2.
Patient-derived cancer cell line preclinical models of EGFR exon 20 insertion mutations to probe mobocertinib and the role of EGFR-C797S as a mechanism of resistance. (A) Dose-response proliferation assays (proportional percent viability) of mobocertinib for all tested patient-derived EGFR-mutated lung adenocarcinoma cell lines. The main driver, EGFR mutation, is indicated for each cell. (B) Western blotting of cell line BID019 (EGFR- N771_H772insH). Cells were treated with the EGFR TKI mobocertinib for 8 hours at the indicated ascending concentrations. pEGFR at position 1068, total EGFR, and β-actin as a loading control are displayed. (C) Relative fold increase in the IC50 of EGFR-L858R+C797S compared with EGFR-L858R in the Ba/F3 system. Cells were plated at a density of 20,000 cells per well (96-well plates) and grown over 3 days after treatment. Three separate experiments were used to generate IC50s for each EGFR TKI tested, erlotinib, osimertinib, and mobocertinib. IC50, 50% inhibitory concentration; pEGFR, phosphorylated EGFR; TKI, tyrosine kinase inhibitor.