Figure 5. The mitochondrial-derived peptide MOTS-c coordinates mitoribosomal stress response in POMC neurons.
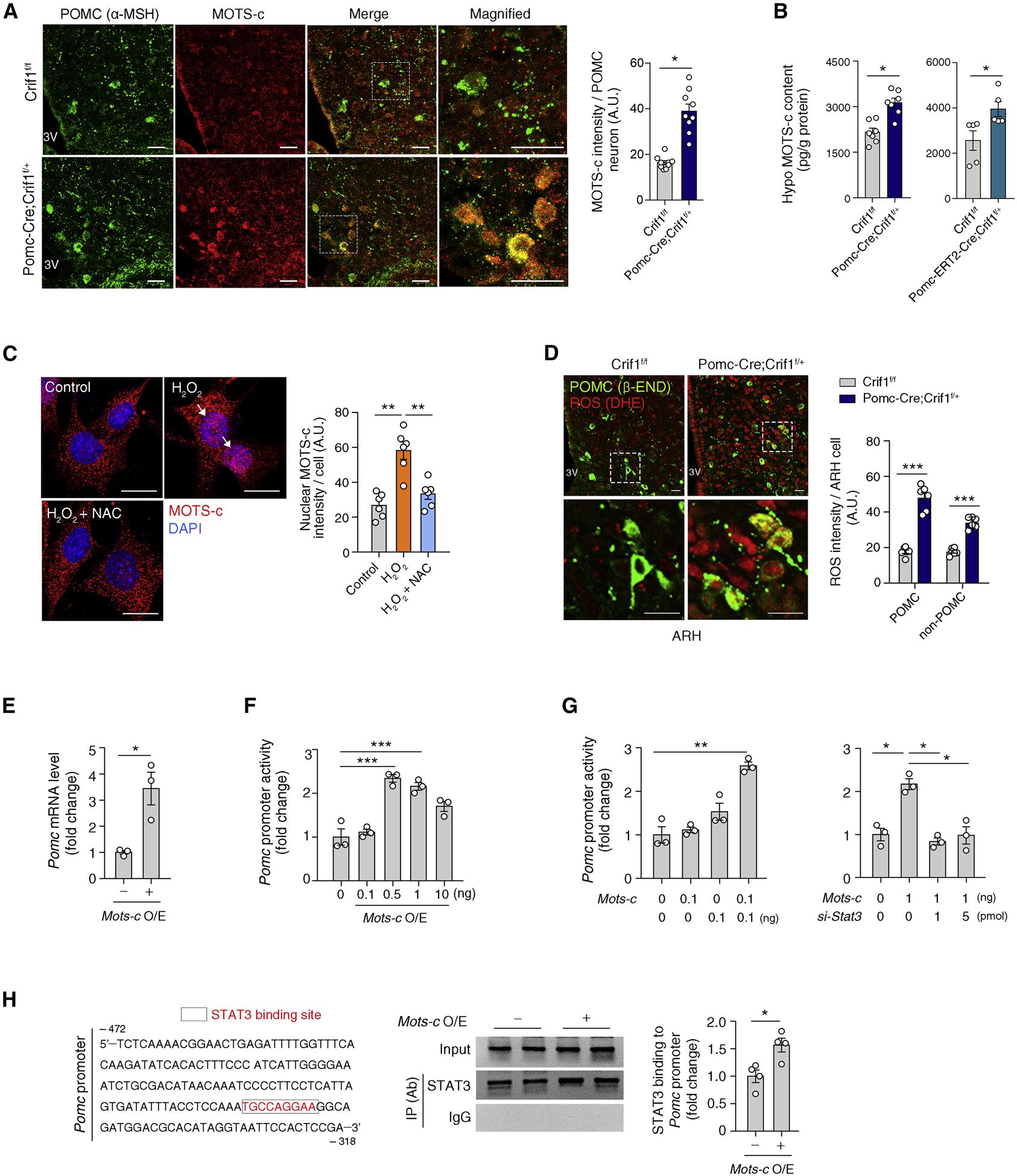
(A) αMSH and MOTS-c double immunohistochemistry showing increased MOTS-c expression in the POMC neurons of Pomc-Cre; Crif1f/+ mice compared to Crif1f/f controls (n = 9). Scale bars, 25 μm.
(B) Increased hypothalamic MOTS-c contents in Pomc-Cre; Crif1f/+ mice and Pomc-ERT2-Cre; Crif1f/+ mice compared to Crif1f/f mice (n = 5–7).
(C) Oxidative stress (H2O2 treatment) induces MOTS-c nuclear localization (white arrows) which is blocked by a cotreatment with antioxidant N-acetylcysteine (NAC) in N1 hypothalamic neurons (n = 6 wells). Scale bars, 25 μm.
(D) Dihydroethidium (DHE) and β-END double staining showing increased ROS production in the ARH of Pomc-Cre; Crif1f/+ mice (n = 6). Scale bars, 10 μm.
(E, F) Effect of Mots-c overexpression (O/E) on Pomc mRNA and Pomc transcriptional activity (n = 3).
(G) Effects of Stat3 coexpression and depletion via siRNA on the MOTS-c-mediated regulation of Pomc transcription (n = 3).
(H) Chromatic immunoprecipitation assay showing increased STAT3 binding to the Pomc promotor area under conditions of Mots-c overexpression (n = 4) IP, immunoprecipitation; Ab, antibody.
Results are presented as a mean ± SEM. *p < 0.05, **p < 0.01, and ***p < 0.001 between indicated groups. See also Figure S5
