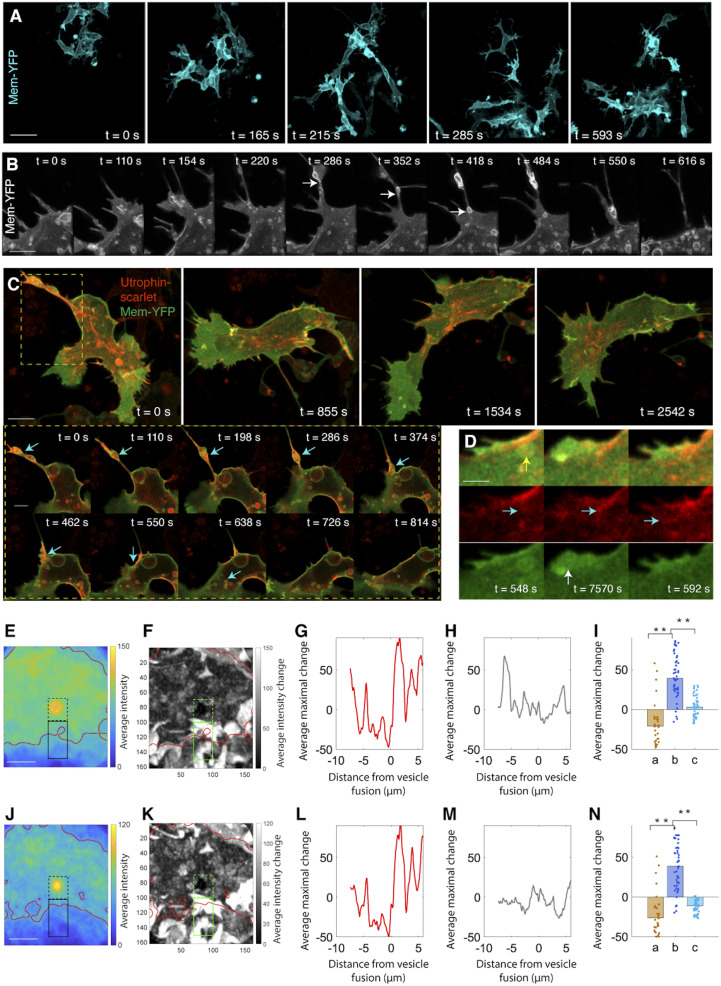
Fig. 4.
Type I vesicles are noncanonical macropinosomes transporting F-actin to the lamellipodium. (A) Live imaging in a 612 × 612 × 13 μm3 region between the dorsal neural tube and the notochord fails to detect evidence of phagocytosis. (Scale bar: 20 µm.) (B) Live imaging of macropinocytosis. The extending protrusion in the cell body retracts and translates into vesicles (arrows). (Scale bar: 3 µm.) (C) Live imaging on macropinosome wrapping of F-actin and subsequent transportation. (Scale bar: 4 µm). Box: zoom in view of the process of cortical actin breakage into actin patches, association with lipid vesicles and transportation into the lamellipodium (arrows) (Scale bar: 2 µm). (D) Addition of a F-actin patch into the actin bundles in the lamellipodium (green: Membrane-YFP; red: Utrophin-scarlet). When a vesicle moves to cell front (yellow arrow), its lipid causes membrane protrusion (white arrow); simultaneously, the actin patch inside this vesicle is released (cyan arrows) and merged with the existing actin bundles. (Scale bar: 1 µm.) (E–N) Morphological analysis confirms lipid expansion of cell membrane (E–I) and F-actin insertion into the lamellipodium (J–N). The same approach in Fig. 2 I–M is applied to assess the contribution of lipid and F-actin to lamellipodial morphogenesis. Focusing on I, increase of Membrane-YFP intensity in the extracellular region (b) suggests that lipid fusion causes membrane protrusion. Similarly, in N, increase of Utrophin-scarlet intensity in the extracellular region is a sign of F-actin insertion into the lamellipodium (for I and N, rank sum test, **P < 0.001, n = 31 pixels representing the average of 17 vesicles). (Scale bars in E and J: 4 µm.)