Fig. 2.
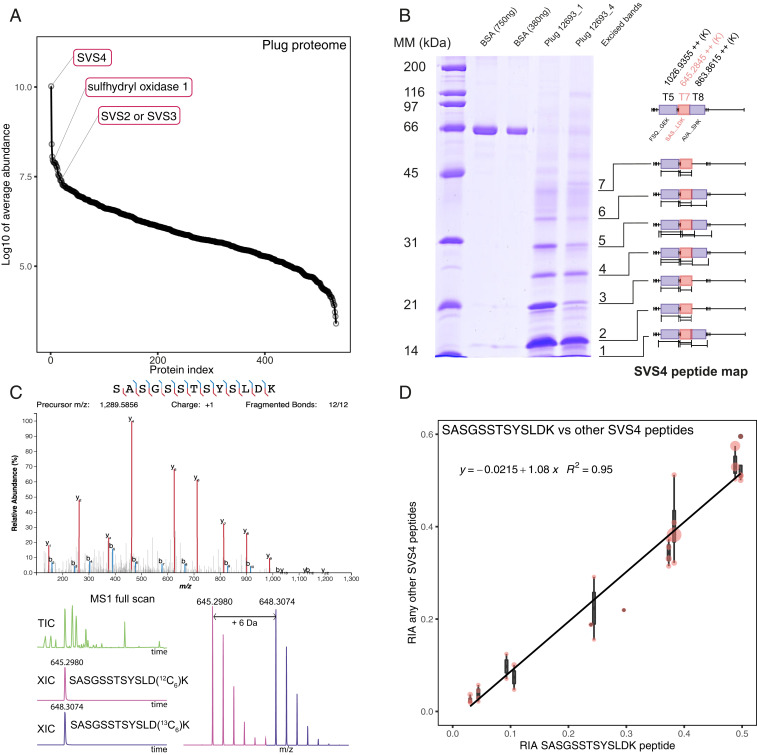
Assessment of mating plugs by peptide mass spectrometry. When plugs were analyzed by proteomics, over 500 proteins were identified. However, the abundance distribution (assessed by label-free quantification) indicates that one protein in particular, SVS4, was over two orders of magnitude more abundant than other proteins (A). Mating plugs from bank voles exhibit a simple pattern on SDS/PAGE, consistent with a polymeric series of a low molecular weight protein (B). This was confirmed by in-gel digests—relevant shaded, boxed peptides from each gel band confirm the primary protein is SVS4 (B). One peptide, SASGSSTSYSLDK (pink in B), confirmed by a complete set of product ions, yielded very strong signals and low noise mass spectra in every plug sample, permitting accurate assignment of plug origin (C). Although this “index” peptide was the strongest ion and was seen in every plug sample (probably because of the absence of cross-linking sites), other peptides in SVS4 were also measured, and the calculation of RIA from those peptides, relative to the index peptide (D), was highly correlated (see also SI Appendix, Fig. S1). Abbreviations: MM, molecular weight markers; BSA, bovine serum albumin; TIC, total ion chromotogram; XIC, extracted ion chromatogram.