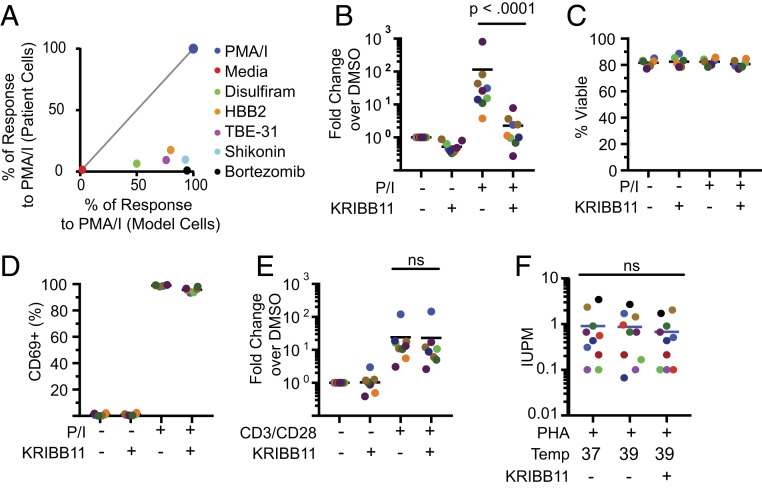
Fig. 3.
Analysis of latency reversal ex vivo in cells from patients on ART. (A) Compounds that induce cellular stress responses reverse latency in a primary cell model but not in resting CD4+ T cells from treated patients. Identical treatment conditions were used for the primary cell model and for resting CD4+ T cells from infected individuals. Graph shows a correlation plot for responses of the two cell types to each stimulus. Latency reversal in the primary cell model was measured using GFP expression. Latency reversal in patient cells was measured by RT-qPCR as described above. In both cases, responses are expressed as the percentage of the positive control response to PMA/I. (B) Effect of KRIBB11 on latency reversal by PMA/I in resting CD4+ T cells from patients on ART (n = 9). Cells were stimulated with PMA/I for 24 h in the presence or absence of 5 µM KRIBB11 and then assayed for HIV-1 transcripts by RT-qPCR. Colored symbols indicate different patients. Statistical significance was determined using ratio-paired t tests. (C) Effect of KRIBB11 on cell viability. Viability of treated primary cells from B was measured with flow cytometry using a vital dye. (D) Effect of KRIBB11 on induction of CD69 expression by PMA/I. Levels of CD69 on cells from B were measured by flow cytometry. (E) Effect of KRIBB11 on latency reversal by anti-CD3/CD28 in resting CD4+ T cells from patients on ART (n = 9). Cells were stimulated with CD3/CD28 stimulation for 72 h in the presence or absence of 5 µM KRIBB11 and then assayed for production of HIV-1 transcripts by RT-qPCR. Statistical significance was determined using ratio-paired t tests. (F) Effect of elevated temperature and KRIBB11 on latency reversal by PHA and allogenic stimulation. Primary resting CD4+ T cells were assayed with a 21-d quantitative viral outgrowth assay at different temperatures in the presence or absence of 5 µM KRIBB11. Data are presented as IUPM.