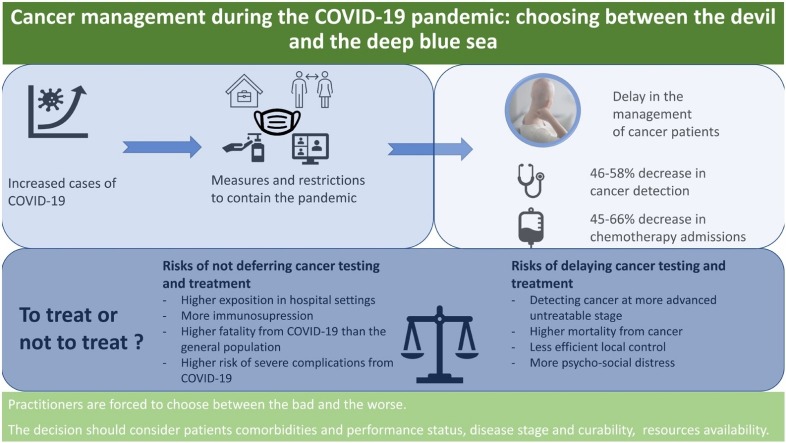
Graphical abstract
Keywords: Cancer, COVID-19, Delay, Oncology, Outcomes, Postpone, Screening, Treatment
Abstract
COVID-19 was declared a “Public Health Emergency of International Concern” in March 2020. Since then, drastic measures were implemented to reduce the virus spread. These measures prevented cancer patients from receiving prompt medical care. A delay in testing and treating cancer patients is thought to protect them from serious COVID-19 complications but exposes them at the same time to the risk of disease progression and cancer related mortality. Healthcare providers are therefore facing the dilemma of choosing between two unpleasant scenarios. To shed light upon the matter, we present in this review article, based on an extensive search of the literature, an overview of the delay in the management of cancer patients, possible contributors to this delay and its benefits and risks on cancer patients’ health.
1. Introduction
Cancer is a major burden on the medical community worldwide. Each year, tens of millions of people are diagnosed with cancer, and more than half of the patients eventually die from it. In many countries, cancer ranks second in general causes of death following cardiovascular diseases. According to the WHO (World Health Organization) “one in five men and one in six women worldwide develop cancer during their lifetime, killing one in eight and one in eleven, respectively” (WHO, 2021).
The first case of coronavirus disease 2019 (COVID-19) was identified in Wuhan, China in late 2019, and the disease has now spread worldwide to be declared “Public Health Emergency of International Concern (PHEIC)” by the WHO in March 2020. In an attempt to contain the COVID-19 pandemic, lockdowns and drastic measures have been implemented in all parts of the word (Carli et al., 2020). Nevertheless, these initiatives have had substantial collateral damage on unrelated, non-COVID-19, medical issues. Several studies have shown that cancer healthcare has been greatly affected in face of the COVID-19 pandemic (Al-Quteimat and Amer, 2020). As a matter of fact, the American Society of Clinical Oncology recommends “to conserve health system resources and reduce patient contact with health care facilities” (Anon., 2021).
This said, the pandemic appears to have a greatly deteriorating impact on cancer patients. Because of cancer’s worsening nature, postponing treatment or screening has considerable potential to increase the likelihood of disease progression to more fatal stages and result in metastasis.
Consequently, oncologists are facing an unfortunate dilemma: choosing to postpone would likely aggravate the disease; but choosing not to do so would expose patients to a potential viral infection leading to equally catastrophic outcomes.
To shed more light on the matter, we conducted a systematic review combining data from several findings in order to pinpoint the consequences of either string of action, followed by a review of available recommendations and guidelines.
2. Methods
The primary objective of this review is to provide insight for physicians of what would be the consequence of either postponing or proceeding with cancer screening and treatment during the pandemic. Another purpose is to propose some important criteria that physicians might look into while making their decision.
To achieve these objectives, an extensive electronic search of the literature was conducted in the PubMed database until the 29th of September 2020. The following keywords with Boolean operators were used “cancer”, “tumor”, “malignancy” in combination with “COVID-19”, “SARS-CoV-2”, “coronavirus” and “outcome”, “death rate”, “ICU admission rate”, “mechanical ventilation”, “delay” and “postponed”. Titles and abstracts of retrieved articles were screened for eligibility, and then entire texts were analyzed and 136 papers that responded to our objectives were included in this review.
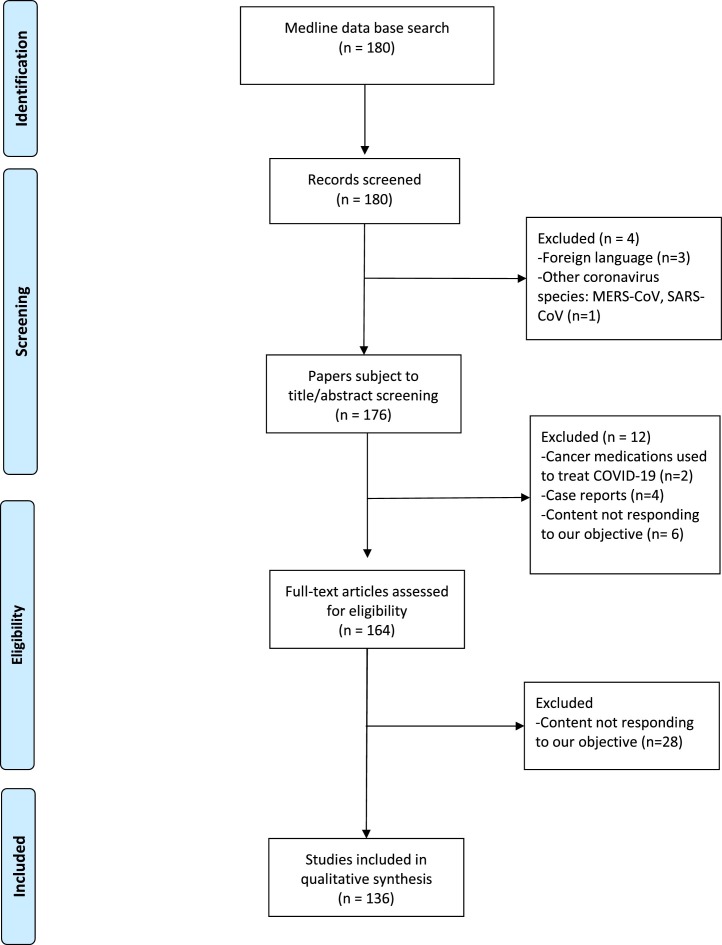
To assess the impact of the delay during the COVID-19 pandemic, only peer-reviewed papers were considered. All papers describing or reporting a delay in cancer screening, diagnosis or treatment during the COVID-19 pandemic as well as papers discussing the impact of this delay on disease control, survival, or mental health of cancer patients were considered eligible. We included review articles, research articles, cohort studies and case series. Reports on coronavirus infections other than COVID-19 and articles concerning the use of cancer medications to treat COVID-19 were excluded. Articles not responding to our primary objective and articles written in a foreign language were similarly omitted. Case report articles were also excluded, as their result cannot be extrapolated to larger population. The PRISMA flow diagram (Fig. 1 ) below depicts the steps of qualitative synthesis of evidence from the literature that this systematic review article was based on.
Fig. 1.
PRISMA flow diagram of the steps of qualitative synthesis of evidence from the literature.
3. Results
As events unfold during this pandemic, lockdown has proved to be a crucial initiative in order to control the wide spread of COVID-19 (Carli et al., 2020). However, the current measures taken to control the spread could in reality impair other areas of the healthcare system. The clearest illustration of this claim is patients with cancer, either diagnosed or awaiting diagnosis, being “bystander victims” of the delay caused by the overload of the healthcare system.
We will present in this work an overview of 15 articles that provide evidence that the delay in the management of cancer patients is real and 13 articles that propose factors contributing to this delay. The impact of this delay on cancer pateints’ health will also be addressed. 11 papers found in the literature propose that postponing investigations and treatment protects cancer patients whereas 35 articles warn from the risk of delaying cancer screeing and treatment. Of note, the articles extracted have different countries of origin, meaning that the pandemic impacted cancer patients over the six continents.
3.1. An overview of the current delay in cancer screening and treatment
The major repercussion on cancer patients is the inability to receive necessary medical services (Wang and Zhang, 2020). The first aspect of cancer that has proven to be significantly affected is screening. Knowing that early detection is a key factor in cancer prognosis, a delay in cancer screening would inevitably have a profound impact on the patients’ health condition. Nonetheless, several studies have reported a depletion in cancer screening since the beginning of the outbreak. For example, Kaufman et al. reported a 46.4 % decrease in weekly cases of cancer diagnosed between March 1 and April 18, 2020 (Kaufman et al., 2020). Uniformly, Amit Sud et al. commented that, due to the pandemic, “2 weeks urgent referral pathways” (which is a request from a General Practitioner to ask the hospital for an urgent appointment in view of symptoms that might indicate cancer) has seen a notable decrease, as much as 84 % (Sud et al., 2020a).
Importantly, this delay was felt all over the world; for instance, the Netherlands Cancer Registry has seen a decline of as much as 40 % in weekly cancer incidence, likewise, the United Kingdom endured a 75 % decline in referrals for suspected cancer since COVID-19 restrictions were implemented (IJzerman and Emery, 2020). Similarly, an 8% median decrease in cancer patients volume was reported across 15 Latin American countries (Martinez et al., 2020).
Moreover, not only one type of cancer was affected by lower screening rates. In the U.S, screening rates for breast, colon, and cervical cancers fell as low as 94 % in March 2020 from their pre-pandemic average (Routine cancer screenings have plummeted during the pandemic, 2020). In Hospital of Philadelphia, Ding et al. reported that they did not see any patients with a new leukemia diagnosis for 35 days, despite a five‐year historical mean of 2.96 days between new leukemia patients (Ding et al., 2020). Notably, a multidisciplinary group, on behalf of European Breast Cancer Research Association of Surgical Trialists (EUBREAST) reported that 20 % of institutions have experienced larger intervals between diagnosis and initiation of treatment (Gasparri et al., 2020).
More so, lower cancer detection rates are to be expected following this decrease in screening: Rutter et al. approximated a 58 % decrease in weekly number of cancers detected, ranging from 19 % in pancreatobiliary cancer to up to 72 % for colorectal cancer (Rutter et al., 2020). Therefore, this delay will probably have repercussion in terms of increased incidence of cancer cases in the near future.
On the other hand, the pandemic has also affected treatment of previously diagnosed cases. In the Middle East, North Africa, and West Asia region, essential treatments like chemotherapy, surgery, and radiation therapy were delayed in 29%–44% of centers (Saab et al., 2020). Lai et al. reported a 45–66% reduction in admission to chemotherapy compared to pre-pandemic settings in 8 hospitals across England and Northern Ireland (Lai et al., 2020). Similarly, Wise reported that chemotherapy appointments were reduced by 60 % in the U.K (Wise, 2020), and Trehan et al. reported that only about one‐third of patients are attending for their appointments (Trehan et al., 2020). However, it is important to note that this is not true in all parts of the words. For instance, in a tertiary hospital in Beirut, Lebanon, according to a survey conducted among cancer patients who receive their chemotherapy regularly at the same-day unit, most of the patients insisted on getting their scheduled treatment without any delay despite their fear of exposure to COVID-19 in the hospital setting and no decrease in admission rates to the same-day unit was noted (Kattan et al., 2021).
In terms of surgical management of cancer patients, a survey conducted in metropolitan Detroit estimated that approximately 72 % of the responding urologists cancelled prostate cancer surgery in more than half of unfavorable intermediate risk cases and 56.2 % have cancelled more than half of high-risk cases (Domenig et al., 2020). Dursun et al. observed that surgeons have decided to change their surgical approach and/or radicality of surgery in 10 % of the cases due to the pandemic. A global predictive model estimated that during the peak phase of the pandemic, 2 324 069 cancer surgery procedures would be postponed globally (COVIDSurg Collaborative, 2020).
The different aspects of the delay in the management of cancer patients are summarized in Table 1 .
Table 1.
Different aspects of the delay in cancer management during the COVID-19 pandemic.
| Aspect of the delay | Evidence of the delay | Region | Reference |
|---|---|---|---|
| Delay in screening | 46.4 % decrease in weekly cases of cancer diagnosed | United States | Kaufman et al., 2020 |
| 84 % decrease in “2 weeks urgent referral pathways” | United Kingdom | Sud et al., 2020a,b | |
| 94 % decrease in screening rates for breast, colon and cervical cancer | United States | Rebecca Robbins, 2020 | |
| 58 % decrease in weekly number of cancers detected | United Kingdom | Rutter et al., 2020 | |
| Delay in medical treatment | 45–66% reduction in admission to chemotherapy | England and Northern Ireland | Lai et al., 2020 |
| 60 % reduction in chemotherapy appointments | United Kingdom | Wise, 2020 | |
| Delay in surgical treatment | 56.2–72% reduction in prostate cancer surgery | United States | de Marinis el al., 2020 |
| 10 % modification in surgical approach | Multiples countries | CovidSurg Collaborative, 2020 |
3.2. Factors leading to the delay in cancer treatment
The COVID-19 pandemic was responsible for a delay in both cancer screening and treatment for many reasons. It is important to note, however, that some countries were more than others, especially middle to low-income countries, given that alternative treatments and/or screening methods in these countries could not always be implemented in order to maintain a continuation of cancer care (Belkacemi et al., 2020).
First, much health care resources are being reallocated to address severe acute respiratory syndrome coronavirus (SARS-CoV-2) infected patients and contain the outbreak (Dinmohamed et al., 2020). This is especially true in hospitals serving populations with a high caseload of COVID-19 such as in New Delhi, Mumbai, Milan, Madrid, and New York, where routine activities such as cancer screening and therapy where held up (de Las Heras et al., 2020). In point of fact, surgical treatment of cancer patients utilizes substantial resources including diagnostic modalities, blood products, ICU beds and even ventilators and personal protective equipment (PPE) (Shinde et al., 2020). Consequently, 70 % of centers experienced a shortage in blood products and 47%–62% of medication shortage was reported in 34 centers from 19 countries in the Middle East, North Africa, and West Asia Region (Saab et al., 2020).
On top of reallocation of these resources to manage COVID-19, fewer volunteers were available for blood donation due to the lockdown. Some donors were also rejected due to their history of exposure to the virus (Shinde et al., 2020).
Shortage of staff was also a contributor to the delay in cancer diagnosis and management, with the mobilization of healthcare providers and physicians to reinforce frontline pandemic care (Wang and Zhang, 2020), along with the isolation of infected or exposed staff members and their unavailability for several days (de C. Zequi and Abreu, 2020).
In addition, additional fees of screening for COVID‐19 as well as funds to supply hospital staff with supplementary PPE kits have contributed to higher cost of cancer treatment, especially in surgical departments (Greco et al., 2020). This must be seen as an important factor leading to a delayed management of cancer. Bakkar et al. reported that half of the participants in their cohort study were subject to an extra personal cost of $1 410 per patient (Bakkar et al., 2020), at a time where financial resources are of utmost importance. In fact, because of the economic downturn created by the pandemic, quite a few Americans had lost their job-provided health insurance coverage and are tightly budgeting how they spend their available family funds (DuBois, 2020).
Notwithstanding, patients themselves also play a major role in promoting the delay. Individuals with potential, non-specific cancer symptoms might postpone seeking treatment and screening whether it is due to concerns on the safety of close contact with medical staff (Dinmohamed et al., 2020; Greco et al., 2020; Fleseriu et al., 2020), or owning to a moral concern of wasting the practitioners’ time while the healthcare system is depleted of resources (Dinmohamed et al., 2020).
Another possible cause is commuting and transportation issues. Unavailability of public transport is a key factor that bankrolled the delay (Greco et al., 2020). For instance, some countries like India have prohibited public transport while only 20 % of patients lived within 50 km of the hospital (Trehan et al., 2020).
Last but not least, this delay in managing cancer patients was also favored by healthcare centers that opted to avoid the exposure of both their medical staff and the patients to the virus. On one hand, surgeons were concerned about operating asymptomatic COVID-19 positive cases, which could wreak havoc in hospitals, and expose healthcare providers and their families to the risk of COVID-19 (Dursun et al., 2020). On the other hand, given that cancer population is more vulnerable to a potential COVID-19 infection, healthcare center recommended avoiding hospital visits whenever possible as a safety measure, leading to a decline in cancer screening and cancer treatment (Wang and Zhang, 2020).
A summary of factors contributing to the delay in cancer management is provided in Table 2 .
Table 2.
Factors leading to the delay in cancer treatment.
| Factors leading to the delay in cancer treatment | |
|---|---|
| Resource-related factors | Reallocation of health care resources to address SARS-CoV-2 |
| Higher costs of treatment per patient to cover PPE provision | |
| Shortage of staff members | |
| Institutional measures to minimize contact between patients and staff members | |
| Factors related to lockdown measures | Unavailability of blood donors |
| Difficulty in accessing healthcare facilities | |
| Patient-related factors | Concerns on the safety of exposure to healthcare professionals |
| Concerns on wasting practitioner’s time amidst the pandemic | |
3.3. The risks of not deferring cancer testing and treatment during the pandemic
A myriad of studies has demonstrated that cancer patients infected with COVID-19 have significantly worse outcome than the general population; therefore, some authors propose to avoid any unnecessary exposure to the virus in order to avoid serious complications:
First, compared to the general population, COVID-19 patients with cancer displayed higher case fatality rate than the general population (5.6 % vs 2.3 %) (Wu and McGoogan, 2020; Deng et al., 2020; T. N. C. P. E. R. E. Team, 2020). Similarly, cancer patients had a high mortality rate (28.6 %) (Zhang et al., 2020). According to a multivariate regression model, Dai et al. proved that cancer was a risk factor of mortality from COVID-19, but their result was not statistically significant (OR = 2.17; (p = 0.06)) (Dai et al., 2020). Deng et al. estimated a relative risk of mortality of 2.926 [1.34–6.41]; (p = 0.006) in cancer patients (Deng et al., 2020). Another meta-analysis of 33 studies demonstrated that all-cause mortality was higher in cancer patients with a relative risk of 1.66 [1.33–2.07]; (p < 0.0001) (Giannakoulis et al., 2020).
Similarly, higher risk of severe events (intensive care unit admission, invasive ventilation requirement or death) was recorded for COVID-19 patients who had cancer (39 % compared to 8% in the general population). In point of fact, “cancer history represented the highest risk for severe events” with an odds ratio of 5.34 [1.80–16.18]; (p = 0.003) (Liang et al., 2020). Dai et al. confirmed that compared to non-cancer cases, cancer patients had worse COVID-19 outcomes, with higher risk of developing more than one critical symptom (OR = 1.99; (p < 0.01)), higher risk of ICU admission (OR=3.13; (p < 0.01)) and higher risk of needing invasive mechanical ventilation (OR= 2.71; (p= 0.037)) (Dai et al., 2020). Giannakoulis et al. also reported a higher ICU admission rate in patients with cancer (RR = 1.56 [1.31–1.87]; (p < 0.0001)) (Giannakoulis et al., 2020).
Furthermore, cancer patients’ health deteriorated more quickly with a COVID-19 infection than non-cancer patients. Liang et al. reported a median time to severe events in cancer patients of 13 days [IQR, 6–15] compared to 43 days [IQR, 20–not reached] (HR = 3.56 [1.65–7.69]; (p < 0.0001)) in patients who do not have cancer (Liang et al., 2020). Likewise, Dai et al. reported a mean length of stay of 27.01 days (SD 9.52) in cancer patients who got infected with COVID-19 compared to 17.75 days (SD 8.64) in non-cancer patients which, according to the Wilcoxon test is statistically significant (p < 0.01) (Dai et al., 2020).
Worse outcomes in cancer patients infected with COVID-19 were explained in the literature by many factors:
-
-
Immune suppression in cancer patients
To begin, cancer by itself can be correlated with lower immunity. Cancer is associated with an increased expression of CD4 and CD25 Treg cells, which are responsible for suppressing patient’s immune cells (Li et al., 2014). Likewise, patients with hematologic malignancies can have malignant or dysfunctional plasma cells, lymphocytes, or white blood cells and therefore have lower immunologic capabilities (Dai et al., 2020).
Furthermore, cancer treatment can cause lower immunity; for example, intensive chemotherapy is associated with an increased risk of bacterial superinfection, resistance to antiviral medication, persistent shedding, long term deterioration of pulmonary function, and pneumonia (Kamboj and Sepkowitz, 2009). Zhang et al. supported this claim and demonstrated that 83.3 % of cancer patients who received their last antitumor treatment within the last 14 days and got infected with SARS-CoV-2 experienced severe events, and were at higher risk of severe event than non-cancerous patients (HR = 4.079 [1.086–15.322]; (p = 0.037)) (Zhang et al., 2020). Similarly, Dai et al. showed that patient who were treated with immunotherapy and got infected with SARS-CoV-2 afterwards had a remarkable death rate of 33.33 % and a 66.67 % risk of contracting severe symptoms. Likewise, patients who underwent cancer surgery had a recorded death rate of 25 % and a risk of developing severe events of 62.5 % (Dai et al., 2020).
-
-
Frequent hospital visits and higher virus exposure
Because cancer patients visit hospitals more frequently, they are at higher risk of contracting the virus. Ning et al. estimated that each patient receiving radiotherapy had an exposure risk to a median of 5 employees (IQR, 3–6.5) through prolonged close contact (Ning et al., 2020). More so, Dai et al. estimated that the proportion of COVID-19 hospital infection was significantly higher in cancer patients than in non-cancer patients (19.04 % vs 1.49 %) (Dai et al., 2020).
-
-
Delayed diagnosis of COVID-19 due to similarity with underlying disease
In lung cancer particularly, symptoms of the disease often overlap with COVID-19 (cough, shortness of breath), potentially causing a delay in diagnosing COVID-19 and more severe symptoms on presentation, leading some practitioners to be more prudent in administering anti-cancer therapy for these patients. Moreover, radiographic findings of COVID-19 may be indistinguishable from pneumonitis caused by lung cancer therapeutics, including immunotherapy, radiotherapy, and oral tyrosine kinase inhibitors (TKIs) (Singh et al., 2020).
The above data incited practitioners in many centers across different countries to encourage delaying cancer treatment, whether surgical or medical, to this vulnerable population during the COVID-19 pandemic in an attempt to minimize patients’ exposure to the virus and subsequent complications.
3.4. The risks of delaying cancer testing and treatment during the pandemic
Cancer is an aggressive and fatal disease. Although some data suggest that a delay in offering treatment can protect cancer patients from a dangerous COVID-19 infection, the lives of most oncology patients depend on their ability to receive prompt medical care. Therefore, prolonged screening and treatment delay could potentially have significant adverse sequelae, including higher death rates.
3.4.1. Impact of delaying cancer screening and testing
Screening delay could lead to detection of cancer at later stages, therefore increasing the risk of progression to an untreatable stage.
For instance, Ricciardiello et al. predicted that a 7 to 12-month delay in screening will likely result in a significant increase of stage IV cancer compared to an interval of 0–3 months (from 26 % to 29 %; p = 0.008). Deferral to more than 1 year will lead to up to 33 % progression to advanced stage disease (p < 0.001) (Ricciardiello et al., 2020). Vanni, in one possible scenario, estimated that a 6 months suspension of breast cancer screening will result in 16 250 temporarily missed diagnostics (Vanni et al., 2020). Likewise, Corley et al. showed that a colonoscopy performed after more than 6 months delay was associated with a higher risk of any colorectal cancer and of advanced stage disease. In fact, lesion progression may occur as soon as 7–9 months after diagnosis (Corley et al., 2017). In regard to squamous cell carcinoma, Jensen et al. established that the increase in tumor volume was significantly correlated to the interval between cross-sectional imaging. Hence, an interval of more than 4 weeks between CT scanners showed a measurable progression of the disease in 70 % of patients compared to only 33 % of patients who performed imaging at less than 2 weeks interval. Additionally, the majority of head and neck cancers double in volume within 1–3 months, regardless of their initial size or location (Jensen et al., 2007). It is important to note that some types of cancer like sino-nasal carcinomas and tumors of the anterior skull base remain asymptomatic early during the disease course and are diagnosed at later, incurable stages even in normal pre-pandemic settings. Therefore, even a slight delay may be lethal for patients harboring these tumors during the pandemic (Turri-Zanoni et al., 2020).
3.4.2. Impact of delaying cancer treatment on survival
Treatment delay had significant lower overall survival rate per 14 day-delay (HR = 1.06 [1.05–1.07]; (p < 0.001)) (Grass et al., 2020). Ginsburg et al. estimated an approximative 10 % increase in hazard ratio of all-cause mortality for each month interval between diagnosis and surgery (HR = 1.10 [1.06–1.14]; (p < 0.001)) (Ginsburg et al., 2020). Similarly, a study in Taiwan showed that patients who received treatment between 90 and 180 days after diagnosis had more than 30 % risk of death than those who received treatment within 90 days (HR = 1.33; [1.02–1.72]; (p < 0.05)) (Chen et al., 2019). SÁNCHEZ-ORTIZ et al. established that patients who experienced a delay from diagnosis to surgery longer than 12 weeks had worse 3-year estimated survival: 34.9 % (+/-13.5 %) vs 62.1 % (+/-4.5 %) in patients undergoing surgery within 12 weeks (HR = 2.51 [1.30–4.83]; (p = 0.006)) (Sánchez-Ortiz et al., 2003).
Moreover, some studies have reported death risks and estimated the impact of delaying treatment on specific types of malignancies:
-
-
In colorectal cancer, a longer time to colonoscopy after an abnormal fecal immunochemical test (FIT) was proven to be associated with a higher risk of advanced stage disease (Balzora et al., 2020). Larson et al. estimated that a 4-month delay of surgery was associated with higher death rate in stage I to III colon cancer (37 % vs 25 % if the surgery was performed within 1 month after diagnostic) (Larson et al., 2020). In like manner, Meester et al. concluded that every additional month from clinical suspicion until colonoscopy was associated with a 0.3 % increase in cancer incidence and a 1.4 % increased risk of mortality (Meester et al., 2016).
-
-
Regarding breast cancer, a time from diagnosis to surgery of more than 90 days lowered overall survival by 3.1–4.6% (Vanni et al., 2020). Moreover, the added risk of death from all causes for each 30 additional days to surgery was 10.0 % (HR = 1.10 [1.07–1.13]; (p < 0.001)) and breast cancer-specific mortality increased with each 60 days interval (sub hazard ratio [sHR]=1.26 [1.02–1.54]; (p = 0.03)) (Bleicher et al., 2016).
-
-
In cutaneous melanoma, Conic et al. demonstrated a higher risk of death when the treatment was received between 90 and 119 days after biopsy compared to when it was done within 30 days of biopsy (HR = 1.09 [1.01–1.18]; (p = 0.03)) (Conic et al., 2018). Likewise, five-year overall survival was notably lower in patients who underwent surgery after more than 60 days following diagnosis, compared to patients with time to surgery less than 30 days (72.7 % vs 80 %) (Basnet et al., 2018).
-
-
As regards cervical cancer, patients who received treatment between 90 and 180 days after diagnosis had a 1.33 times higher risk of death than those who received treatment within 90 days (Chen et al., 2019).
-
-
As for penile cancer, 5-year specific survival significantly decreased due to a 3-month delay in the treatment of lymph nodes (39.5 % vs 64.1 % without delay) (Méjean et al., 2020).
Cancer patients are therefore bystander victims of the pandemic, and a higher death rate would be expected due to a delay in receiving an appropriate treatment. Furthermore, the Imperial College model estimates that 6 months are necessary to stop the spread of COVID-19 (Vanni et al., 2020). Based on this, many models attempted to expect mortality rates that arise from delaying treatment. Some data estimate that a uniform 6-month delay in cancer cases diagnosed via the “2-week urgent pathway” at stage I–III will cause an additional 9280 deaths and 173 540 life years lost within the following decade in the U.K. Additionally, a 6-month delay is predicted to result in more than 30 % decrease in long-term (10 year) survival (Sud et al., 2020a). Another study in the U.K estimated that a 6 months surgical delay per patient is likely to result in additional 10 760 deaths and 208 275 life-years lost (Sud et al., 2020b). In addition, Wise estimated that over the span of a year, 6270 additional deaths in England (which corresponds to a 20 % increase in death rate) and 33 890 additional deaths in the U.S are to be expected in patients with new cancer diagnosis because of the COVID-19 pandemic (Wise, 2020).
In parallel, Lai et al.’s model estimated that under a Relative Impact of the Emergency (RIE) of 1.5 and with 40 % of the population being affected by the emergency, 17 915 more deaths are to be expected across incident and prevalent cancer cases within a year in England (Lai et al., 2020). In like manner, Larson et al. predicted that a delay of 4 months will result in 10 043 additional deaths over a span of 5 years, in colon cancer alone (Larson et al., 2020). Maringe estimated that within 5 years, due to the delayed treatment of breast, colorectal, lung and esophageal cancer, 3291–3621 additional deaths and 59 204 to 63 229 life-years lost are to be anticipated in the U.K. (Maringe et al., 2020) Similarly, an extra 10 000 deaths (the equivalent of a 1% increase) are expected in breast cancer and colorectal cancer, over the next decade in the U.S alone (Sharpless, 2020).
3.4.3. Impact of delaying cancer treatment on disease control
Delaying treatment for cancer patients not only affects their survival but can also lead to harder-to-treat stages of the disease.
Nugent et al. showed that a delay of more than 56 days in treatment of cervical cancer was associated with an increased risk of pelvic failure over the span of 3 years (26 % vs 9% within 56 days) (p = 0.04) (Song et al., 2013). Perez et al. also noted a 0.37 % loss of control per day in patients with stage IB and IIA pelvic cancer, and a 0.68 % loss of control per day in Stage IIB (Perez et al., 1995). Similarly, Keane et al. estimated a 1% loss of control per day of delay for early stage cervical cancer (Keane et al., 1992).
Comparably, it was estimated that every day of delay in treatment was associated with a decrease in local control of 2.7 % for tonsil cancer (Keane et al., 1992), 1.4 % for laryngeal cancer (Barton et al., 1992) and 1% for oropharyngeal cancer (Bentzenh et al., 1991). Moreover, patients with Merkel cell carcinoma waiting a median of 24 days for radiotherapy experienced a high rate of disease progression (41 %) (Tsang et al., 2004).
It is important to note that particularly in radiation therapy, postponing treatment will make future management more complicated. For instance, Barton et al. estimated a dose increment of 0.64 Gy/day in order to maintain isoeffective local control for daily fractions of 2.5 Gy (Barton et al., 1992). Similarly, Bentzenh et al. recorded an additional mean required dose of 0.68 Gy [IQR, 0.05–1.31] to compensate one day of treatment delay (Bentzenh et al., 1991).
3.4.4. Impact of delaying cancer treatment on patient’s mental health
It is worth mentioning that delaying cancer treatment has shown to not only impact patients’ physical health but also their mental health. In fact, cancer patients are prone to engender psychiatric diseases such as anxiety and depression. Adding to this, lockdown and isolation imposed by the emerging of COVID-19 have created a sense of collective hysteria, and delaying treatment could be an important factor of increased stress (Cancarevic et al., 2020). In other words, constant fear and uncertainty caused by the pandemic could harm cancer patients’ quality of life (Chakraborty and Pandey, 2020), and this was illustrated by more than one survey in cancer population.
In an online survey, 71 % of the cancer population felt that they are at higher risk of COVID-19 infection than the general population, which has resulted in a higher anxiety index related to COVID-19 (Staehler et al., 2020). Another online questionnaire that targeted breast cancer patients reported that 8.9 % of the participants experienced severe anxiety and 9.3 % suffered from severe depression since the beginning of the pandemic, while previous studies reported only 3.5 % of severe anxiety and depression in breast cancer patients (Juanjuan et al., 2020). In a different questionnaire, 86 % of the oncologic patients scheduled for major surgery who filled the questionnaire reported loss of energy and increased anxiety and depression. Additionally, 20.8 % felt to be under severe distress and 4% reported suffering from insomnia (Greco et al., 2020). In a like manner, 41 % of the participants in a survey designed for sarcoma patients reported that their emotional wellbeing has been affected in the COVID-19 period (Younger et al., 2020). Bakkar et al. reported that a delay in receiving conventional radioactive iodine ablation placed thyroid cancer patients in the mild-to-moderate anxiety group according to the HAM-A scale. Importantly, those who could not afford an additional cost of therapy had to cope with a higher burden of stress (Bakkar et al., 2020).
4. Discussion
In the present article, we investigated on many levels the consequence of the COVID-19 pandemic on cancer patients. It is well established that this disease resulted in cancer screening and treatment delays. The available evidence suggests that in case of a potential COVID-19 infection, worse outcomes are recorded in cancer patients, including higher death rates and higher risk of complications. On the other hand, the delay can have serious repercussions on cancer patients in terms of disease curability and patients’ survival with more eventual life-years loss than can cause a COVID-19 infection. Therefore, practitioners are facing the dilemma whether or not to defer testing and treatment.
For this purpose, we propose some guidelines and a framework to guide decisions on delaying screening and treatment on the basis of available data and experts’ opinion:
In general, decision should be based on a case-by-case analysis (Del Pilar Estevez-Diz et al., 2020). For that reason, it is favored that multidisciplinary board continues to take place, preferentially using teleconference systems (Turri-Zanoni et al., 2020; Sundriyal et al., 2020; Tan et al., 2020). Physicians must weigh the patient's risk of COVID‐19 complications in the event of exposure against the risk of worse oncologic outcomes from delaying cancer therapy (Baumann et al., 2020). A triage system should be implemented accordingly in each hospital and health care setting. Obviously, one size does not fit all, and guidelines vary according to cancer stage and histology. Either way, delay in diagnosis is not advised and tumor grading and staging should be done as soon as possible to guide the management (Turri-Zanoni et al., 2020).
Several articles have provided insight into how practitioners should approach this difficult dilemma accordingly to each cancer histology. Suggestions and guidelines are available for head and neck, sino-nasal, spinal, breast, upper tract urothelial, cervical, penile, testicular, pancreatic, kidney, gastric, colorectal, skin, adrenal, liver, prostate, ovarian, endometrial, bladder and endocrine cancer (Cohen et al., 2020; Baumann et al., 2020; Werner et al., 2020; Tarantola et al., 2013; Catanese et al., 2020; Vecchione et al., 2020; Turri-Zanoni et al., 2020; de C. Zequi and Abreu, 2020; Gravas et al., 2020; O’Leary et al., 2020; Casco et al., 2020; Katims et al., 2020; Berjano et al., 2020; Kapuria et al., 2020; P. March 25 and 2020, 2021P. March 25 and 2020, 2021; Tachibana et al., 2020; Loveday et al., 2020; Jones et al., 2020; Villani et al., 2020; Fligor et al., 2020; Sanchez et al., 2020; Wallis et al., 2020; Topf et al., 2020; Matsuo et al., 2020; Colombo et al., 2020; Campi et al., 2020; Raghavan et al., 2020).
Briefly, the decision of tolerating a delay in treatment is based on the risk of disease progression. For example, treatment of early-stage breast cancer, low or intermediate-risk prostate cancer, low-grade lymphoma, non-melanoma skin cancer, and other low-risk cancer diseases could be delayed in a safe manner for over 3 months. In contrary, high grade and aggressive types of cancers like colon cancer with obstruction, ovarian, liver, or pancreatic tumors and small cell lung cancer must be treated as soon as the diagnosis is made given that delay in treating these types of cancer is associated with an elevated risk of disease progression (Al-Quteimat and Amer, 2020). Besides, prompt cancer care is to be provided to patients with potentially curable disease without any delay (Turri-Zanoni et al., 2020).
The cut-off and threshold of delaying cancer treatment and screening will also vary based on geographical capabilities and local burden of COVID-19 cases and the phase of the pandemic (Everett et al., 2020).
It is crucial that restrictive measures in determined regions be categorized throughout the pandemic according to local protocols, taking into consideration the level of contagion locally (Mauri et al., 2020).
Age and comorbid conditions are also factors of paramount importance. Although Zhang et al. first reported an increased risk of complications in patients who recently received anti-cancer therapy, their cohort consisted of only 28 patients (Zhang et al., 2020). Pinato et al. noted in a multi-national observational study of 890 patients that pursuing the application of systemic anti-cancer therapy is not an independent factor of mortality by itself. Treatment does not compromise outcomes if administered using a sorting process which demonstrates the hierarchy in severity of the cases and taking into account other adverse risk factors, especially age and comorbidity. In fact, younger patients without comorbidities are at lower risk of complications and mortality, and anticancer treatment should be prioritized in this group. On the contrary, elderly and multiple comorbidity patients are at higher risk of long-term complications and mortality; therefore their treatment would better be withheld because they don't have the same chances of survival (Pinato et al., 2020). Also, Amit Sud et al. noted that with a high risk of infection (≥2·5% per referral), the risk associated with investigatory referral in patients older than 70 years might exceed the absolute survival benefit for tumor-referral. Contrariwise, for patients who are 60 years old and younger, delay-related cancer fatality rate largely exceeds investigation-related fatality and therefore a delay is not recommended (Sud et al., 2020a).
Telemedicine could be in many instances a good option and may be used to advise patients for proper therapy and symptom control measures, as well as to advise new patients regarding diagnostic tests (Bhatla and Singhal, 2020). A systematic review on the proficiency of telemedicine found that this communication method appears to be a good alternative with its applicability, ease and cost-effectiveness being supported by various publications: 61 % of the studies found telehealth less costly than the non-telehealth alternative and 33 % of studies found improved health outcomes thanks to telemedicine (Wade et al., 2010).
A national network and regional collaboration with other cancer centers can help reduce the backlog and reduce patients’ risk of infection by sending them to less impacted cities (Lee et al., 2020; Larson et al., 2020). Gupta & Sharma proposed the creation of specialized ‘cancer hubs’, maintained as COVID-19-free, where cancer patients from multiple tumor groups are fast tracked on priority basis (Gupta and Sharma, 2020).
De Almeida et al. developed and tested a prioritization and ranking algorithm, called SPARTAN‐HN (Surgical Prioritization and Ranking Tool and Navigation Aid for Head and Neck Cancer) which consistently stratifies patients needing surgery for head and neck cancer. This algorithm demonstrated excellent concurrence and correlation with expert rankings and might be even a better and faster way of stratifying cancer cases (de Almeida et al., 2020). Similarly, Chhabra et al. developed an internal algorithm that helped maintain patients’ treatment volume while keeping the rate of COVID-19 infection acceptable. Importantly, this algorithm considers treatment-related, tumor-related, and patient-related characteristics in order to guide the safest decision (Chhabra et al., 2020).
Additionally, resources that are needed in surgery are being reallocated for the treatment of COVID-19. Besides, post-operative hospitalization is mandatory, making surgery a less desired treatment method. Therefore, consideration should be given for postponing non-critical procedures and promoting non-surgical treatment whenever possible (Larson et al., 2020). Surgeons should also be grouped into small cross-functional teams to ensure continuity of services in the event that a patient tests positive for SARS-CoV-2 (Tan et al., 2020). However, it is important to note that delaying surgery is not always possible, especially in low to middle-income countries where radiation therapy facilities for example are not largely available and therefore not to the reach of everyone (Del Pilar Estevez-Diz et al., 2020).
Concerning radiation therapy, moderately hypo-fractionated radiotherapy can be considered in some cases to minimize the frequency of hospital visits (Cimbak and Barker, 2016).
Other screening alternatives are also coming to light: Moul proposed a novel exosome-based urine test (ExosomeDx; Bio-Techne) which is sent to the patients by mail in an attempt to replace the in-person visit digital rectal examination (Moul, 2020).
More so, oral administration of targeted therapies for the treatment of some types of cancer such as lung cancer may be an appealing alternative in the current crisis, allowing patients to receive their full therapy while lowering hospital visits and minimizing exposure (Jonna et al., 2020).
importantly, because the patient's mental health depends on how the message is being transmitted, health care professionals should emphasize the need to correctly address the patient, especially in these challenging times (Gharzai et al., 2020).
We respectfully agree that our work was not without limitations. It was not possible to draw evident conclusions to guide the decision on proceeding with screening and treatment for cancer patients during the pandemic for many reasons.
First, studies that assess the outcomes of cancer patients with COVID-19 are of small sample size; death rates and severe events could be therefore higher or lower. However, the results we could extract from the major studies are significant despite the low sample size. We believe that these results should be interpreted with caution in the absence of adjustment to the prevalence of cancer in the general population.
Second, most of the studies that discourage delaying treatment of cancer patients are not performed in the era of COVID-19. In fact, their data suggest that delaying treatments can impact disease control and patients’ survival in general, but very few data directly compare this risk against that of contracting a COVID-19 infection. Therefore, their conclusions cannot be extrapolated to populations at high risk of exposure to this disease. On the other hand, most of the studies that support postponing testing and treatment to protect cancer patients from an unnecessary exposure were performed at the beginning of the pandemic. Those studies did not take into account the possibility of an extended duration of the pandemic and the consequences of a prolonged period without any treatment for the underlying disease.
Another major limitation is that most of the recommendations we exposed are based on experts’ opinions, and higher levels of evidence are needed to draw fruitful conclusions.
5. Conclusion
The delay in providing appropriate care for cancer patients is real, and many factors have contributed to it: the shortage of hospital-based resources and their reallocation for the management of COVID-19, the limited capacity of health care facilities, patients’ anxiety towards a minacious COVID-19 infection, and the crowding of hospitals by COVID-19 cases. In fact, differing cancer screening leads to the detection of the tumor at harder-to-treat stages, and postponing treatment can cost lower cure rates, higher death rates and more life-years loss. In parallel, cancer screening and follow up make patients more exposed to the virus; and offering medical or surgical care puts them in a more immunocompromised state with more susceptibility to complications. This has its toll on the patients’ mental health, which makes the recovery path longer and tougher.
In front of this dilemma, it is choosing between the bad and the worse, and this decision is a burdensome task that should not come without a great deal of consideration beforehand, taking into account the situation of each patient and all the influencing factors. Should this healthcare need be neglected, cancer patients and the healthcare system will only pay the price later with mortality, morbidity, and financial burden.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgement
None.
Biographies
Marc Boutros: Medical Student Saint Joseph University, Faculty of Medicine, Beirut, Lebanon.
Elissar Moujaess: Hematology and Medical Oncology fellow at Hotel Dieu de France Hospital, Beirut, Lebanon. Graduated from Saint Joseph University, Faculty of Medicine, Beirut, Lebanon.
Hampig Raphael Kourie: Hematologist and Medical Oncologist at Hotel Dieu de France Hospital, affiliated to Saint Joseph University, Faculty of Medicine, Beirut, Lebanon.
References
- Al-Quteimat O.M., Amer A.M. The impact of the COVID-19 pandemic on cancer patients. Am. J. Clin. Oncol. 2020 doi: 10.1097/COC.0000000000000712. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anon . ASCO; 2021. Cancer Screening, Diagnosis, Staging &; Surveillance.https://www.asco.org/asco-coronavirus-resources/care-individuals-cancer-during-covid-19/cancer-screening-diagnosis-staging [Google Scholar]
- Bakkar S., et al. Impact of COVID-19 on thyroid cancer surgery and adjunct therapy. Updates Surg. 2020;72(September 3):867–869. doi: 10.1007/s13304-020-00833-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzora S., Issaka R.B., Anyane-Yeboa A., Gray D.M., May F.P. Impact of COVID-19 on colorectal cancer disparities and the way forward. Gastrointest. Endosc. 2020;92(4):946–950. doi: 10.1016/j.gie.2020.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton M.B., Keane T.J., Gadalla T., Maki E. The effect of treatment time and treatment interruption on tumour control following radical radiotherapy of laryngeal cancer. Radiother. Oncol. 1992;23(March 3):137–143. doi: 10.1016/0167-8140(92)90323-M. [DOI] [PubMed] [Google Scholar]
- Basnet A., Wang D., Sinha S., Sivapiragasam A. Effect of a delay in definitive surgery in melanoma on overall survival: a NCDB analysis. JCO. 2018;36(May 15_suppl) doi: 10.1200/JCO.2018.36.15_suppl.e21586. pp. e21586–e21586. [DOI] [Google Scholar]
- Baumann B.C., et al. Management of primary skin cancer during a pandemic: multidisciplinary recommendations. Cancer. 2020;126(17):3900–3906. doi: 10.1002/cncr.32969. 01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkacemi Y., et al. A review of the international early recommendations for departments organization and cancer management priorities during the global COVID-19 pandemic: applicability in low- and middle-income countries. Eur. J. Cancer. 2020;135:130–146. doi: 10.1016/j.ejca.2020.05.015. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzenh S.M., Johansen L.V., Overgaard J., Thames H.D. Clinical radiobiology of squamous cell carcinoma of the oropharynx. Int. J. Radiat. Oncol. Biol. Phys. 1991;20(June 6):1197–1206. doi: 10.1016/0360-3016(91)90228-V. [DOI] [PubMed] [Google Scholar]
- Berjano P., Vanni D., Fariselli L., Cecchinato R., Boriani S. Strategy for the practice of spine oncological surgery during the Covid-19 pandemic. Spine. 2020;45(October 19):1386–1394. doi: 10.1097/BRS.0000000000003623. [DOI] [PubMed] [Google Scholar]
- Bhatla N., Singhal S. The COVID-19 pandemic and implications for gynaecologic cancer care. Indian J Gynecol Oncol. 2020;18(2):48. doi: 10.1007/s40944-020-00395-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleicher R.J., et al. Time to surgery and breast cancer survival in the United States. JAMA Oncol. 2016;2(March 3):330–339. doi: 10.1001/jamaoncol.2015.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campi R., et al. Assessing the burden of nondeferrable major uro-oncologic surgery to guide prioritisation strategies during the COVID-19 pandemic: insights from three italian high-volume referral centres. Eur. Urol. 2020;78(July 1):11–15. doi: 10.1016/j.eururo.2020.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancarevic I., Tathineni P., Malik B.H. Coronavirus disease 2019 (COVID-19) in cancer patients. Cureus. 2020;12(April 4) doi: 10.7759/cureus.7835. p. e7835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carli R., Cavone G., Epicoco N., Scarabaggio P., Dotoli M. Model predictive control to mitigate the COVID-19 outbreak in a multi-region scenario. Annu. Rev. Control. 2020 doi: 10.1016/j.arcontrol.2020.09.005. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casco N.C., Carmona M.J., Soto Á.J. Therapeutic and surgical indications for patients with penile cancer in the COVID-19 era. Int. Braz. J. Urol. 2020;46(suppl.1):86–92. doi: 10.1590/S1677-5538.IBJU.2020.S110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catanese S., Pentheroudakis G., Douillard J.-Y., Lordick F. ESMO management and treatment adapted recommendations in the COVID-19 era: pancreatic cancer. ESMO Open. 2020;5(Suppl 3) doi: 10.1136/esmoopen-2020-000804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty M., Pandey M. Caring for cancer patients in the Covid pandemic: choosing between the devil and deep sea. World J. Surg. Oncol. 2020;18(August 1) doi: 10.1186/s12957-020-02002-7. p. 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.-P., Kung P.-T., Wang Y.-H., Tsai W.-C. Effect of time interval from diagnosis to treatment for cervical cancer on survival: a nationwide cohort study. PLoS One. 2019;14(September 9) doi: 10.1371/journal.pone.0221946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabra A.M., Choi J.I., Hasan S., Press R.H., Simone C.B. Prioritization of proton patients in the COVID-19 pandemic: recommendations from the New York Proton Center. Int. J. Part. Ther. 2020;6(4):38–44. doi: 10.14338/IJPT-20-00022.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimbak N., Barker C.A. Short-course radiation therapy for Merkel cell carcinoma: relative effectiveness in a ‘Radiosensitive’ tumor. Int. J. Radiat. Oncol. Biol. Phys. 2016;96(October 2) doi: 10.1016/j.ijrobp.2016.06.387. p. S160. [DOI] [Google Scholar]
- Cohen M.A., Powell A.M., Coleman J.S., Keller J.M., Livingston A., Anderson J.R. Special ambulatory gynecologic considerations in the era of coronavirus disease 2019 (COVID-19) and implications for future practice. Am. J. Obstet. Gynecol. 2020;223(3):372–378. doi: 10.1016/j.ajog.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo I., et al. ESMO management and treatment adapted recommendations in the COVID-19 era: gynaecological malignancies. ESMO Open. 2020;5(Suppl 3) doi: 10.1136/esmoopen-2020-000827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conic R.Z., Cabrera C.I., Khorana A.A., Gastman B.R. Determination of the impact of melanoma surgical timing on survival using the National cancer database. J. Am. Acad. Dermatol. 2018;78(January 1):40–46. doi: 10.1016/j.jaad.2017.08.039. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corley D.A., et al. Association between time to colonoscopy after a positive fecal test and risk of colorectal cancer stage at diagnosis. JAMA. 2017;317(April 16):1631–1641. doi: 10.1001/jama.2017.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVIDSurg Collaborative Elective surgery cancellations due to the COVID-19 pandemic: global predictive modelling to inform surgical recovery plans. BJS (Br. J. Surg.) 2020;107(11):1440–1449. doi: 10.1002/bjs.11746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai M., et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10(6):783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida J.R., et al. Development and validation of a surgical prioritization and ranking tool and navigation aid for head and neck cancer (SPARTAN-HN) in a scarce resource setting: response to the COVID-19 pandemic. Cancer. 2020 doi: 10.1002/cncr.33114. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de C. Zequi S., Abreu D. Consideration in the management of renal cell carcinoma during the COVID-19 Pandemic. Int. Braz. J. Urol. 2020;46(suppl.1):69–78. doi: 10.1590/S1677-5538.IBJU.2020.S108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Las Heras B., et al. Cancer treatment and research during the COVID-19 pandemic: experience of the first 6 months. Oncol. Ther. 2020 doi: 10.1007/s40487-020-00124-2. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Pilar Estevez-Diz M., Bonadio R.C., Miranda V.C., Carvalho J.P. Management of cervical cancer patients during the COVID-19 pandemic: a challenge for developing countries. Ecancermedicalscience. 2020;14 doi: 10.3332/ecancer.2020.1060. p. 1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng G., Yin M., Chen X., Zeng F. Clinical determinants for fatality of 44,672 patients with COVID-19. Crit. Care. 2020 doi: 10.1186/s13054-020-02902-w. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y.-Y., et al. Delayed cancer diagnoses and high mortality in children during the COVID-19 pandemic. Pediatr. Blood Cancer. 2020 doi: 10.1002/pbc.28427. p. e28427, Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinmohamed A.G., et al. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020;21(6):750–751. doi: 10.1016/S1470-2045(20)30265-5. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenig P., et al. Management of prostate cancer during COVID-19 pandemic: perspective from urologists and radiation oncologists in COVID Dense Metro Detroit. Cureus. 2020;12(8) doi: 10.7759/cureus.9648. p. e9648, Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBois R.N. COVID-19, cancer care and prevention. Cancer Prev. Res. (Phila) 2020 doi: 10.1158/1940-6207.CAPR-20-0468. Sep. [DOI] [PubMed] [Google Scholar]
- Dursun P., et al. Performing gynecologic cancer surgery during the COVID-19 pandemic in Turkey: a multicenter retrospective observational study. Int. J. Gynaecol. Obstet. 2020 doi: 10.1002/ijgo.13296. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett A.S., Strickler S., Marcrom S.R., McDonald A.M. Students’ perspectives and concerns for the 2020 to 2021 radiation oncology interview season. Adv. Radiat. Oncol. 2020 doi: 10.1016/j.adro.2020.08.011. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleseriu M., Dekkers O.M., Karavitaki N. Endocrinology in the time of COVID-19: management of pituitary tumours. Eur. J. Endocrinol. 2020;183(July 1):G17–G23. doi: 10.1530/EJE-20-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fligor S.C., et al. Gastrointestinal malignancies and the COVID-19 pandemic: evidence-based triage to surgery. J. Gastrointest. Surg. 2020;24(10):2357–2373. doi: 10.1007/s11605-020-04712-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparri M.L., Gentilini O.D., Lueftner D., Kuehn T., Kaidar-Person O., Poortmans P. Changes in breast cancer management during the corona virus disease 19 pandemic: an international survey of the European Breast Cancer research Association of Surgical Trialists (EUBREAST) Breast. 2020;52:110–115. doi: 10.1016/j.breast.2020.05.006. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharzai L.A., Resnicow K., An L.C., Jagsi R. Perspectives on oncology-specific language during the coronavirus disease 2019 pandemic: a qualitative study. JAMA Oncol. 2020 doi: 10.1001/jamaoncol.2020.2980. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakoulis V.G., Papoutsi E., Siempos I.I. Effect of Cancer on clinical outcomes of patients with COVID-19: a meta-analysis of patient data. JCO Glob. Oncol. 2020;6:799–808. doi: 10.1200/GO.20.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg K.B., et al. Association of surgical delay and overall survival in patients with T2 renal masses: implications for critical clinical decision-making during the COVID-19 pandemic. Urology. 2020 doi: 10.1016/j.urology.2020.09.010. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grass F., et al. Impact of delay to surgery on survival in stage I-III colon cancer. Eur. J. Surg. Oncol. 2020;46(March 3):455–461. doi: 10.1016/j.ejso.2019.11.513. [DOI] [PubMed] [Google Scholar]
- Gravas S., et al. Prioritising urological surgery in the COVID-19 era: a global reflection on guidelines. Eur. Urol. Focus. 2020;6(September 5):1104–1110. doi: 10.1016/j.euf.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco F., Altieri V.M., Esperto F., Mirone V., Scarpa R.M. Impact of COVID-19 pandemic on health-related quality of life in uro-oncologic patients: what should we wait for? Clin. Genitourin. Cancer. 2020 doi: 10.1016/j.clgc.2020.07.008. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Sharma P. Management strategies for cancer patients in the time of COVID-19 pandemic. J. Maxillofac. Oral Surg. 2020;19(September 3):473–474. doi: 10.1007/s12663-020-01402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IJzerman M., Emery J. Is a delayed cancer diagnosis a consequence of COVID-19? Pursuit. 2020 https://pursuit.unimelb.edu.au/articles/is-a-delayed-cancer-diagnosis-a-consequence-of-covid-19 Apr. 30 (Accessed Oct. 05, 2020) [Google Scholar]
- Jensen A.R., Nellemann H.M., Overgaard J. Tumor progression in waiting time for radiotherapy in head and neck cancer. Radiother. Oncol. 2007;84(July 1):5–10. doi: 10.1016/j.radonc.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Jones C.M., et al. Considerations for the treatment of pancreatic cancer during the COVID-19 pandemic: the UK consensus position. Br. J. Cancer. 2020;123(5):709–713. doi: 10.1038/s41416-020-0980-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonna S., Reuss J.E., Kim C., Liu S.V. Oral chemotherapy for treatment of lung cancer. Front. Oncol. 2020;10:793. doi: 10.3389/fonc.2020.00793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juanjuan L., et al. Patient-reported outcomes of patients with breast cancer during the COVID-19 outbreak in the epicenter of China: a cross-sectional survey study. Clin. Breast Cancer. 2020 doi: 10.1016/j.clbc.2020.06.003. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamboj M., Sepkowitz K.A. Nosocomial infections in patients with cancer. Lancet Oncol. 2009;10(June 6):589–597. doi: 10.1016/S1470-2045(09)70069-5. [DOI] [PubMed] [Google Scholar]
- Kapuria D., et al. Roadmap to resuming care for liver diseases after coronavirus disease-2019. J. Gastroenterol. Hepatol. 2020 doi: 10.1111/jgh.15178. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katims A.B., et al. Urologic oncology practice during COVID-19 pandemic: a systematic review on what can be deferrable vs. nondeferrable. Urol. Oncol. 2020;38(10):783–792. doi: 10.1016/j.urolonc.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattan C., Badreddine H., Rassy E., Kourie H.R., Kattan J. The impact of the coronavirus pandemic on the management of cancer patients in Lebanon: a single institutional experience. Future Oncol. 2021 doi: 10.2217/fon-2020-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman H.W., Chen Z., Niles J., Fesko Y. Changes in the number of US patients with newly identified cancer before and during the coronavirus disease 2019 (COVID-19) pandemic. JAMA Netw. Open. 2020;3(August 8) doi: 10.1001/jamanetworkopen.2020.17267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane T.J., Fyles A., O’Sullivan B., Barton M., Maki E., Simm J. The effect of treatment duration on local control of squamous carcinoma of the tonsil and carcinoma of the cervix. Semin. Radiat. Oncol. 1992;2(January 1):26–28. doi: 10.1016/S1053-4296(05)80047-5. [DOI] [Google Scholar]
- Lai A.G., et al. Estimating excess mortality in people with cancer and multimorbidity in the COVID-19 emergency. medRxiv. 2020 doi: 10.1101/2020.05.27.20083287. p. 2020.05.27.20083287, Jun. [DOI] [Google Scholar]
- Larson D.W., Abd El Aziz M.A., Mandrekar J.N. How many lives will delay of colon cancer surgery cost during the COVID-19 pandemic? An analysis based on the US national cancer database. Mayo Clin. Proc. 2020;95(8):1805–1807. doi: 10.1016/j.mayocp.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A.K.F., et al. Mitigation of head and neck cancer service disruption during COVID-19 in Hong Kong through telehealth and multi-institutional collaboration. Head Neck. 2020;42(July 7):1454–1459. doi: 10.1002/hed.26226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.-Y., et al. Selective depletion of regulatory T cell subsets by docetaxel treatment in patients with nonsmall cell lung cancer. J. Immunol. Res. 2014;2014 doi: 10.1155/2014/286170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveday C., et al. Prioritisation by FIT to mitigate the impact of delays in the 2-week wait colorectal cancer referral pathway during the COVID-19 pandemic: a UK modelling study. Gut. 2020 doi: 10.1136/gutjnl-2020-321650. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maringe C., et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21(8):1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D., et al. COVID’s impact on radiation oncology: a Latin American survey study. Int. J. Radiat. Oncol. Biol. Phys. 2020;108(October 2):374–378. doi: 10.1016/j.ijrobp.2020.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo K., et al. Wait-time for hysterectomy and survival of women with early-stage cervical cancer: a clinical implication during the coronavirus pandemic. Gynecol. Oncol. 2020;158(1):37–43. doi: 10.1016/j.ygyno.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauri D., et al. Behind the numbers and the panic of a viral pandemic: fixed restrictive oncology guidance may jeopardize patients’ survival. J. BUON. 2020;25(June 3):1277–1280. [PubMed] [Google Scholar]
- Meester R.G.S., et al. Consequences of increasing time to colonoscopy examination following positive result from fecal colorectal cancer screening test. Clin. Gastroenterol. Hepatol. 2016;14(October 10):1445–1451. doi: 10.1016/j.cgh.2016.05.017. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méjean A., et al. Recommandations CCAFU sur la prise en charge des cancers de l’appareil urogénital en période d’épidémie au coronavirus COVID-19. Prog. Urol. 2020;30(April 5):221–231. doi: 10.1016/j.purol.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moul J.W. Are COVID-19 delays a basis for concern? Can we use this for future good? Oncology (Williston Park, N.Y.) 2020;34(9):344–345. doi: 10.46883/ONC.2020.3409.0344.1. 15. [DOI] [PubMed] [Google Scholar]
- Ning M.S., et al. Mitigating the impact of COVID-19 on oncology: clinical and operational lessons from a prospective radiation oncology cohort tested for COVID-19. Radiother. Oncol. 2020;148:252–257. doi: 10.1016/j.radonc.2020.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary M.P., Choong K.C., Thornblade L.W., Fakih M.G., Fong Y., Kaiser A.M. Management considerations for the surgical treatment of colorectal cancer during the global Covid-19 pandemic. Ann. Surg. 2020;272(2):e98–e105. doi: 10.1097/SLA.0000000000004029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- P. March 25 and 2020 Recommendations for prioritization, treatment and triage of breast cancer patients during the COVID-19 pandemic: executive summary. Am. Coll. Surg. 2021 doi: 10.1007/s10549-020-05644-z. https://www.facs.org/quality-programs/cancer/executive-summary (Accessed Oct. 30, 2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez C.A., Grigsby P.W., Castro-Vita H., Lockett M.A. Carcinoma of the uterine cervix. I. Impact of prolongation of overall treatment time and timing of brachytherapy on outcome of radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 1995;32(July 5):1275–1288. doi: 10.1016/0360-3016(95)00220-S. [DOI] [PubMed] [Google Scholar]
- Pinato D.J., et al. Clinical portrait of the SARS-CoV-2 epidemic in European patients with cancer. Cancer Discov. 2020;10(October 10):1465. doi: 10.1158/2159-8290.CD-20-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan D., et al. Management changes for patients with endocrine-related cancers in the COVID-19 pandemic. Endocr. Relat. Cancer. 2020;27(9):R357–R374. doi: 10.1530/ERC-20-0229. [DOI] [PubMed] [Google Scholar]
- Ricciardiello L., et al. Impact of SARS-CoV-2 pandemic on colorectal cancer screening delay: effect on stage shift and increased mortality. Clin. Gastroenterol. Hepatol. 2020 doi: 10.1016/j.cgh.2020.09.008. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routine cancer screenings have plummeted during the pandemic . 2020. STAT.https://www.statnews.com/2020/05/04/cancer-screenings-drop-coronavirus-pandemic-epic/ [Google Scholar]
- Rutter M.D., Brookes M., Lee T.J., Rogers P., Sharp L. Impact of the COVID-19 pandemic on UK endoscopic activity and cancer detection: a national endoscopy database analysis. Gut. 2020 doi: 10.1136/gutjnl-2020-322179. Jul. [DOI] [PubMed] [Google Scholar]
- Saab R., et al. Impact of the coronavirus disease 2019 (COVID-19) pandemic on pediatric oncology care in the Middle East, North Africa, and West Asia Region: a report from the Pediatric Oncology East and Mediterranean (POEM) group. Cancer. 2020 doi: 10.1002/cncr.33075. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez L.R., et al. Clinical and surgical assistance in prostate cancer during the COVID-19 pandemic: implementation of assistance protocols. Int. Braz. J. Urol. 2020;46(suppl.1):50–61. doi: 10.1590/S1677-5538.IBJU.2020.S106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Ortiz R.F., Huang W.C., Mick R., Arsdalen K.N.V., Wein A.J., Malkowicz S.B. An interval longer than 12 weeks between the diagnosis of muscle invasion and cystectomy is associated with worse outcome in bladder carcinoma. J. Urol. 2003 doi: 10.1016/S0022-5347%2805%2964047-5. Jan Accessed: Sep. 30, 2020. [Online]. Available: [DOI] [PubMed] [Google Scholar]
- Sharpless N.E. COVID-19 and cancer. Science. 2020;368(June 6497) doi: 10.1126/science.abd3377. pp. 1290–1290. [DOI] [PubMed] [Google Scholar]
- Shinde R.S., et al. To do or not to do?-A review of cancer surgery triage guidelines in COVID-19 pandemic. Indian J. Surg. Oncol. 2020:1–7. doi: 10.1007/s13193-020-01086-7. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.P., et al. Management of lung cancer during the COVID-19 pandemic. JCO Oncol. Pract. 2020;16(September 9):579–586. doi: 10.1200/OP.20.00286. [DOI] [PubMed] [Google Scholar]
- Song S., et al. The effect of treatment time in locally advanced cervical cancer in the era of concurrent chemoradiotherapy. Cancer. 2013;119(2):325–331. doi: 10.1002/cncr.27652. [DOI] [PubMed] [Google Scholar]
- Staehler M., Battle D., Pal S.K., Bergerot C.D. Counterbalancing COVID-19 with cancer surveillance and therapy: a survey of patients with renal cell carcinoma. Eur. Urol. Focus. 2020 doi: 10.1016/j.euf.2020.09.002. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sud A., et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21(8):1035–1044. doi: 10.1016/S1470-2045(20)30392-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sud A., et al. Collateral damage: the impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann. Oncol. 2020;31(8):1065–1074. doi: 10.1016/j.annonc.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundriyal D., Sehrawat A., Kumar P., Bhandari R. Impact of COVID-19 pandemic on oncology practices during nationwide lockdown period: a single centre experience and the way forward. J. Assoc. Phys. India. 2020;68(July 7):48–50. [PubMed] [Google Scholar]
- T. N. C. P. E. R. E. Team The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) — China, 2020. CCDCW. 2020;2(February 8):113–122. doi: 10.46234/ccdcw2020.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana I., et al. Delaying cancer cases in urology during COVID-19: review of the literature. J. Urol. 2020 doi: 10.1097/JU.0000000000001288. p. 101097JU0000000000001288, Aug. [DOI] [PubMed] [Google Scholar]
- Tan K.-K., Moran B.J., Solomon M.J. Avoiding collateral mortality in a pandemic - time to change our mindset in surgical oncology. Nat. Rev. Clin. Oncol. 2020;17(7):383–385. doi: 10.1038/s41571-020-0383-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantola T.I., et al. Prognostic factors in Merkel cell carcinoma: analysis of 240 cases. J. Am. Acad. Dermatol. 2013;68(March 3):425–432. doi: 10.1016/j.jaad.2012.09.036. [DOI] [PubMed] [Google Scholar]
- Topf M.C., et al. Framework for prioritizing head and neck surgery during the COVID-19 pandemic. Head Neck. 2020;42(6):1159–1167. doi: 10.1002/hed.26184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trehan A., Jain R., Bansal D. Oncology care in a lower middle‐income country during the COVID‐19 pandemic. Pediatr. Blood Cancer. 2020;67(August 8) doi: 10.1002/pbc.28438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang G., O’Brien P., Robertson R., Hamilton C., Wratten C., Denham J. All delays before radiotherapy risk progression of Merkel cell carcinoma. Australas. Radiol. 2004;48(3):371–375. doi: 10.1111/j.0004-8461.2004.01321.x. [DOI] [PubMed] [Google Scholar]
- Turri-Zanoni M., Battaglia P., Karligkiotis A., Locatelli D., Castelnuovo P. Managing care for patients with sinonasal and anterior skull base cancers during the COVID-19 pandemic. Head Neck. 2020;42(July 7):1503–1506. doi: 10.1002/hed.26257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanni G., et al. Breast cancer and COVID-19: the effect of fear on patients’ decision-making process. In Vivo. 2020;34(June 3 suppl):1651–1659. doi: 10.21873/invivo.11957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchione L., Stintzing S., Pentheroudakis G., Douillard J.-Y., Lordick F. ESMO management and treatment adapted recommendations in the COVID-19 era: colorectal cancer. ESMO Open. 2020;5(Suppl 3) doi: 10.1136/esmoopen-2020-000826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villani A., Fabbrocini G., Costa C., Scalvenzi M. Melanoma screening days during the coronavirus disease 2019 (COVID-19) pandemic: strategies to adopt. Dermatol. Ther. (Heidelb) 2020;10(August 4):525–527. doi: 10.1007/s13555-020-00402-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade V.A., Karnon J., Elshaug A.G., Hiller J.E. A systematic review of economic analyses of telehealth services using real time video communication. BMC Health Serv. Res. 2010;10 doi: 10.1186/1472-6963-10-233. p. 233, Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis C.J.D., et al. Risks from deferring treatment for genitourinary cancers: a collaborative review to aid triage and management during the COVID-19 pandemic. Eur. Urol. 2020;78(July 1):29–42. doi: 10.1016/j.eururo.2020.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Zhang L. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020;21(April 4):e181. doi: 10.1016/S1470-2045(20)30149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner M.T., Carey R.M., Albergotti W.G., Lukens J.N., Brody R.M. Impact of the COVID-19 pandemic on the management of head and neck malignancies. Otolaryngol. Head. Neck Surg. 2020;162(6):816–817. doi: 10.1177/0194599820921413. [DOI] [PubMed] [Google Scholar]
- WHO . 2021. Latest Global Cancer Data: Cancer burden Rises to 18.1 Million New Cases and 9.6 Million Cancer Deaths in 2018.https://www.who.int/cancer/PRGlobocanFinal.pdf [Online]. Available: [Google Scholar]
- Wise J. Covid-19: cancer mortality could rise at least 20% because of pandemic, study finds. BMJ. 2020;369 doi: 10.1136/bmj.m1735. Apr. [DOI] [PubMed] [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. 07. [DOI] [PubMed] [Google Scholar]
- Younger E., et al. Health-related quality of life and experiences of sarcoma patients during the COVID-19 pandemic. Cancers (Basel) 2020;12(August 8) doi: 10.3390/cancers12082288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann. Oncol. 2020;31(7):894–901. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]