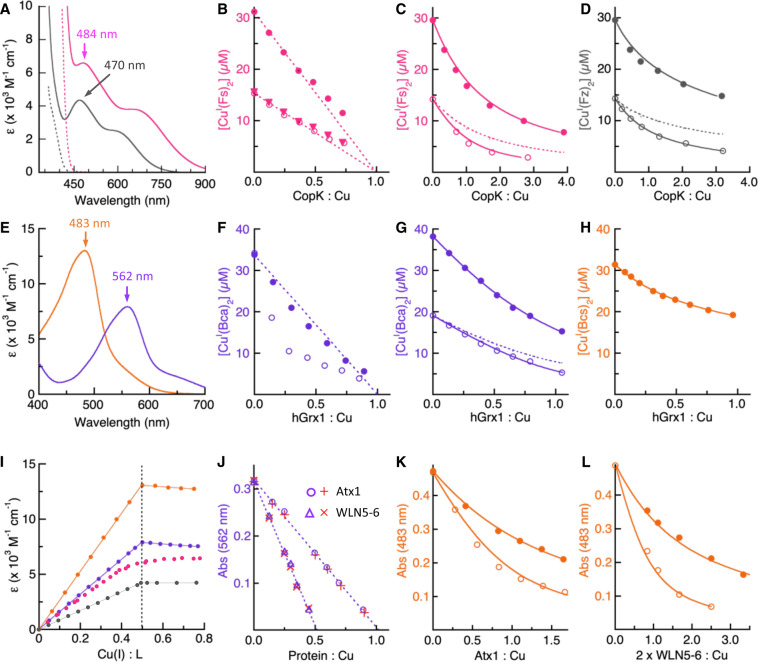
Figure 3. Examples of the flexible application of ‘turn-on’ chromophoric probes CuIL2 (L = Fs, Fz, Bca, Bcs).
(a) Solution spectra of CuI(Fs)2 (magenta) and CuI(Fz)2 (grey) and the respective apo-probe ligands (dashed traces). (b) Determination of Cu(I)-binding stoichiometry of CopK protein with CuI(Fs)2. Change in probe concentrations (monitored by absorbance) with increasing [CopK] in a series of assay solutions (details in Table 2). The data points in triangles show the 50% values of the solid circles, demonstrating that the equilibrium position in each diluted solution has not noticeably adjusted from that present in the corresponding undiluted solution (consistent with stoichiometric binding). It was apparent that CopK could bind one equivalent of Cu(I) with sub-nM affinity. (c,d) Determination of Cu(I)-binding affinity of CopK with CuI(Fs)2 (c) or CuI(Fz)2 (d) (details in Table 2). The solid traces are data fits to equation 14a, deriving KD(P) given in Table 2. The two dashed traces shows the 50% values of the filled circles and demonstrate that the equilibrium position in each diluted solution has adjusted from that present in the corresponding undiluted solution. (e) Solution spectra of CuI(Bca)2 (purple) and CuI(Bcs)2 (orange) (neither probe ligand has absorbance in the given visible window). (f) Determination of Cu(I)-binding stoichiometry of hGrx1 protein with the CuI(Bca)2 probe (details in Table 2). It was apparent that hGrx1 could bind more than one Cu(I) with KD(P) < 10−13 M but only one Cu(I) with KD(P) < 10−14 M at pH 7.0. (g,h) Determination of Cu(I)-binding affinity of hGrx1 with CuI(Bca)2 (g) or CuI(Bcs)2 (h) (details in Table 2). The solid traces are the data fits to equation 14a, deriving KD(P) given in Table 2. The dashed trace in (g) shows the 50% values of the filled circles and demonstrates that the equilibrium position in each diluted solution has adjusted from that present in the corresponding undiluted solution. (i) Cu(I) titration of each probe ligand under reducing conditions followed by absorbance at the λmax (nm) given in (a,e): a tight turning point was observed for ligands Bcs, Bca and Fz, but not for Fs due to its relative weak affinity for Cu(I). Thus, presence of an excess of Fs is essential for its application. (j,k,l) Quantification of Cu(I)-binding to yeast Atx1 and human WLN5–6 in KPi buffer (pH 7.0): (j) determination of respective Cu(I)-binding stoichiometry with two assay solutions of CuI(Bca)2 described in Table 2; determination of aM affinity of Atx1 (k) and WLN5–6 (l) with two different assay solutions of CuI(Bcs)2 described in Table 2. The solid traces are the data fits to equation 14a, deriving KD(P) given in Table 2. Data (a–d, i), (f–h) and (j–l) adapted from refs. [33], [37] and [8], respectively.