The coronavirus disease 2019 (COVID-19) continues to pose unprecedented challenges to patients, physicians, and healthcare setups alike. Its numerous clinical ramifications remain unabated, with increasing medical literature establishing its involvement in the onset and perpetuation of a myriad of extrapulmonary manifestations. Of these extrapulmonary manifestations, hepatic impairment has been extensively reported by researchers and clinicians. While hepatic impairment is noted to be an established possible sequel of a COVID-19 infection, its pathophysiological basis remains unclear [1,2]. In order to devise effective, all-encompassing management protocols, it is imperative to thoroughly elucidate the pathophysiology and clinical manifestations of COVID-19 plays in causing liver injury. Herein, we aim to review the literature to date on hepatic manifestations of COVID-19 in patients with no pre-existing liver disease, patients with chronic liver disease and patients with liver transplantation.
Viral tropism heavily depends on the presence of virus-specific receptors at the membrane of a host cell [3]. For SARS-CoV-2, this is mediated by the virus' spike (S) protein interacting with the ACE-2 and TMPRSS-2 receptors, ultimately resulting in an endocytic cell-entry pathway [3]. Pertinently, the SARS-CoV-2 RNA has been found in extrapulmonary organs such as the liver. However, the cellular sites of replication have yet to be elucidated. The liver injury seen in COVID-19 patients is generally mild, even in the setting of pre-existing liver conditions and in severe COVID-19. The major concern, however, stems from the liver's pivotal role in drug metabolism and core endogenous processes such as coagulation, osmotic pressure maintenance, and acute phase reactant production. Understanding the mechanisms by which SARS-CoV-2 could gain entry into a hepatocyte could therefore prove beneficial for the development of therapeutic targets and management strategies.
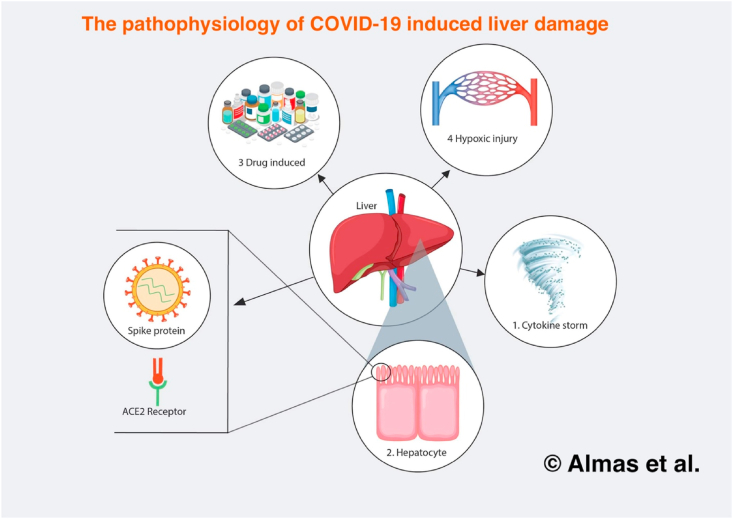
There are three proposed mechanisms which have been found to cause liver damage COVID-19 patients. The first mechanism is through a direct effect of the virus. ACE-2 receptor expression is a major determinant of a cell's susceptibility to SARS-CoV-2 infection [2,4]. The ACE-2 receptors are ubiquitous throughout the body, particularly in type II pneumocytes and the hepatobiliary system [2]. A recent RNA sequencing study found similar levels of ACE-2 expression in cholangiocytes when compared to those in lung alveoli, the primary infection site for COVID-19 [5]. ACE-2 receptors were found to be expressed in 59.7% of cholangiocytes, a proportion similar to that in type II pneumocytes. This insinuates the vulnerability of cholangiocytes to SARS-CoV-2 [2,4,5]. A potential deleterious cytopathic effect of SARS-CoV-2 is biliary tree dysfunction and imminent liver dysfunction [4]. This notion has also been corroborated by the elevated levels of gamma-glutamyl transferase (GGT) observed in several SARS-CoV-2 case series [2].
In healthy hepatocytes, the ACE-2 receptor levels were significantly lower, at 2.6% [5]. However, these levels were elevated in specific circumstances. Firstly, fibrosis and cirrhosis increase ACE-2 receptor expression, supporting the results from previous studies that indicated a higher risk for SARS-CoV-2 hepatocellular tropism in patients with underlying liver injury [3]. Similarly, hypoxia is another circumstance in which ACE-2 expression is upregulated in hepatocytes. This serves as a potential explanation for the dissemination of extrapulmonary manifestations in patients who develop more severe hypoxic COVID-19 symptoms such as ARDS. The possible mechanisms by which COVID-19 causes liver injury are delineated in Fig. 1.
Fig. 1.
The pathophysiology of COVID-19 induced liver damage.
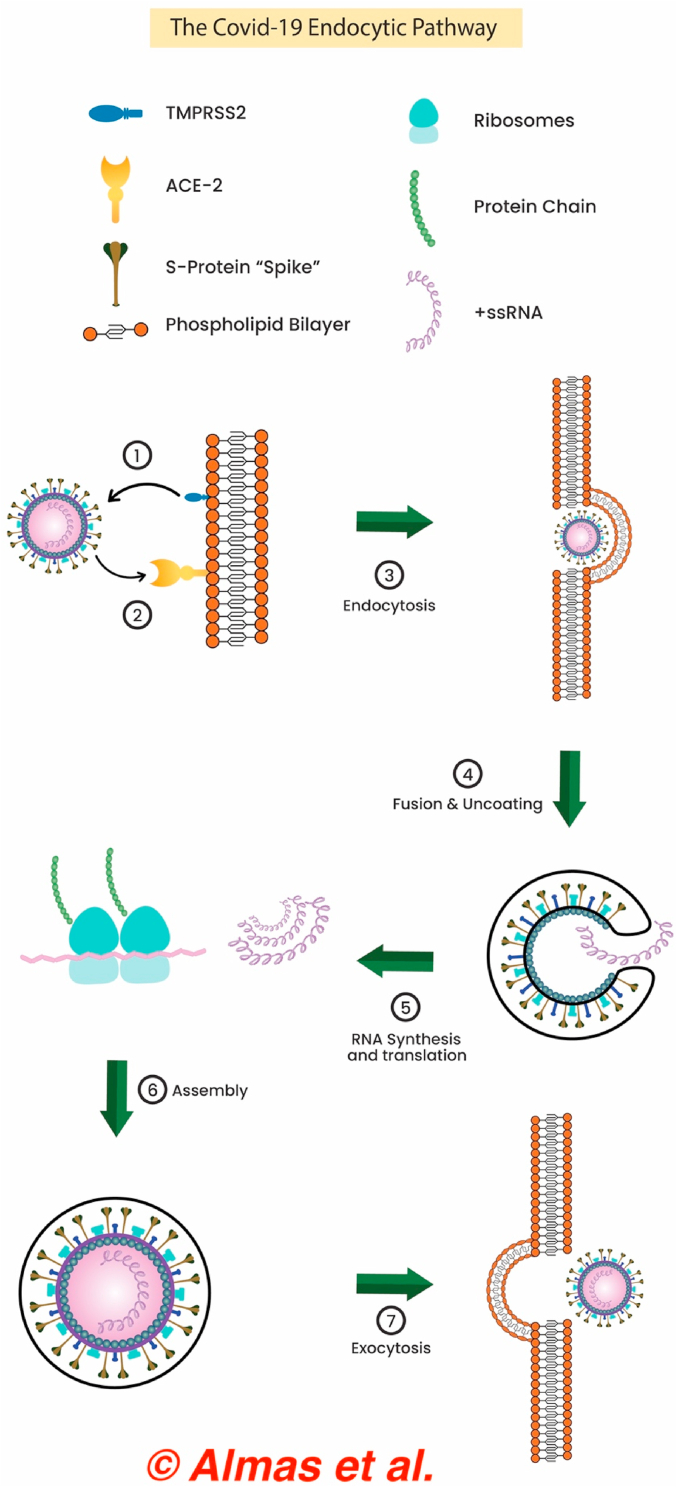
The pathway for viral entry into hepatocyte cell lines might also utilize the PIKfyve-TCP2 endocytic pathway present in the liver and gallbladder at similar levels to the lungs [3]. Other studies have showed that preincubation of the beta-coronavirus’ S protein with trypsin enhanced the affinity for its receptor on the host cell surface. Trypsin, among other proteases, is expressed in the hepatic epithelial cells as they are crucial for remodeling the extracellular matrix. This enhanced affinity for the S protein in the presence of trypsin may be enough to compensate for the lower levels of ACE-2 expression in healthy hepatocytes. An important distinction needs to be made, however, between viral entry into a cell and viral replication—and consequently proliferation—within the cell. Notably, SARS-CoV-2 was able to replicate in the hepatocyte cell line as well as overexpress proinflammatory cytokines that perpetuate underlying hepatic damage [6]. The endocytic pathway is elucidated by Fig. 2 below.
Fig. 2.
The COVID-19 endocytic pathway responsible for hepatic impairment.
SARS-CoV-2 tropism might occur by infecting monocyte-derived macrophages. Since monocyte-derived macrophages are known to express ACE-2 receptors, it is not surprising that lung alveolar macrophages stained positive in immunohistochemical detection of SARS-CoV-2 [3]. However, the specialized hepatic tissue macrophages, Kupffer cells, did not stain; this was despite the fact that Kupffer cells proliferated in the setting of a COVID-19 infection [3]. It was subsequently discovered that Kupffer cells do not express ACE-2 receptors, at least in healthy human livers, from which these RNA sequences were analysed. Reliable comparison calls for similar quantification in COVID-19 patients with underlying liver conditions. When the liver is injured, monocyte-derived macrophages extravasate into the liver. If these immune cells were infected with SARS-CoV-2, they could potentially be carriers to ACE-2 receptor-expressing hepatocytes.
The second mechanism of liver damage is through a dysregulated immune response. Severe COVID-19 patients have an increased activation of inflammatory markers [[2], [3], [4]]. This occurs due to upregulated innate immune response with T-cell lymphopenia [3]. These mechanisms could lead to a cytokine storm, which in turn could lead to pulmonary and extrapulmonary injuries including hepatic inflammation and injury [2]. The overactivation of the immune response leads to an activation of cytotoxic (CD8+) T-cells that survey the body and induce the destruction of virally infected cells. Intracellular molecules released by these apoptosed cells can thus increase inflammatory signals.
The third mechanism of liver damage is through drug-induced toxicity from drugs employed to treat COVID-19. This is especially important in two patient populations: patients with pre-existing chronic liver disease and liver transplant patients. The former are more susceptible to drug toxicity due to impaired drug metabolism. Contrarily, liver transplant patients may be on immunosuppressants that have potential interactions with other drugs [7]. Numerous drugs have been used worldwide in order to treat COVID-19 patients, from antipyretics and steroids to antimalarials and antivirals. Hepatotoxicity has already been proven to be a potential side effect of a number of these drugs, including remdesivir, lopinavir/ritonavir, paracetamol, macrolides, and quinolones [2,3].
Autopsies that were performed on COVID-19 patients with no underlying hepatic conditions revealed steatosis [3]. Multiple studies involving cell lines showed that endoplasmic reticulum (ER) stress markers were significantly elevated during SARS-CoV-2 infection [3]. As ER stress is a major influencing factor for de novo lipogenesis in hepatocytes, SARS-CoV-2 has been implicated in heralding the onset of hepatic steatosis [3]. Compounding this is the mammalian target of rapamycin (mTOR), a regulator of autophagy and inducer of de novo lipogenesis. Cell lines infected with MERS-CoV exhibited hyperactivation of their mTOR signalling pathway; inhibition of mTOR by rapamycin in these infected cell lines inhibited MERS-CoV viral replication [3]. Given the anti-autophagosomal properties of SARS-CoV-2, it might indeed share its mechanism of infection with that of the other coronavirus family members: by direct infection and activation of the mTOR signaling pathway. This is exacerbated by an indirect pathway involving IL-6 stimulation, which further upregulates mTOR activity. As we know SARS-CoV-2 infection upregulates IL-6 production, the resultant cytokine storm effects could lead to even further propagation of mTOR signaling and thus contribute to steatosis.
Hepatic injury in COVID-19 patients has been posited to be a direct consequence of the viral infection. Pathological studies have established the presence of SARS in both liver cells and bile duct cells [8]. Other etiologies of hepatic involvement encompass drug reactions, an aberrant immune response, and hypoxia secondary to COVID-19 induced respiratory compromise [9]. Interestingly, studies from China have reported the presence of liver-related comorbidities in approximately 2–11% of COVID-19 patients [8,9]. Furthermore, 14–53% of COVID-19 cases are noted to present with elevated levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) [10]. The disease severity has also been suspected to correlate strongly with derangements in the ALT/AST levels. Guan et al. noted that AST was elevated in 112 (18.2%) of 615 non-severe patients and 56 (39.4%) of 142 severe patients [11]. Similarly, ALT was elevated in 19.8% non-severe patients and 28.1% severe patients [11]. In another study comprising 5700 patients, 39% of the patients exhibited ALT levels greater than 60 U/L while 58.4% of the patients presented with AST levels greater than 40 U/L [12]. The same study reported that 56 patients (0.98%) of all the participants progressed to acute hepatic injury, as indicated by AST or ALT levels greater than 15 times the reference value, out of which 53 died [12]. However, most studies have reported liver dysfunction due to COVID-19 to be generally mild and transient [13]. Nevertheless, liver dysfunction secondary to a COVID-19 infection has been associated with worse clinical outcomes, with a recent study demonstrating a deteriorating clinical picture in patients with significant hepatic impairment [14].
Chronic liver disease (CLD) is a lifelong pathological process that is characterized by continuous, sustained destruction of the liver parenchyma and its gradual replacement by fibrous tissue, resulting in reduced hepatic function [15]. A meta-analysis found that the prevalence of CLD in COVID-19 patients is 3% [95% CI 0.03, 0.04] [16]. CLD in COVID-19 patients correlated with an exacerbation of COVID symptomatology (pooled OR 1.48 [95% CI 1.17, 1.87], p = 0.001) and a higher overall mortality (pooled OR 1.78 [95% CI 1.09, 2.93], p = 0.02) [16]. In patients with underlying liver disease, the mortality rate was 30–36% [17]. Although it can be argued that there is a low incidence of pre-existing liver pathologies among COVID-19 infections, the high mortality rate should prompt further research into strategizing an altered therapeutic regimen with careful precautions for this small, yet vulnerable, patient group. Clinically, these patients may not tolerate some of the drugs and biological agents routinely used to treat a COVID-19 infection. Drugs such as baricitinib and tocilizumab, for instance, could potentially reactivate conditions like hepatitis B virus (HBV) infection. Similarly, the characteristic hepatotoxicity that occurs due to the uptake of a COVID-19 pharmacological regimen is a major concern for patients with CLD [3]. Lastly, it is possible that SARS-CoV-2 itself exacerbates any underlying liver disease by compromising the remaining functional liver tissue during an ongoing infection.
Another major concern with SARS-CoV-2 was the danger it posed to immunosuppressed patient populations, of which liver transplant (LT) patients make up one of the largest groups [18]. In one large, multicenter study examining the clinical characteristics and outcomes of 126 LT and 43,508 non-LT patients, adjusted analysis showed that 40% of the patients in the LT group required hospitalization compared to 23% in non-LT (risk ratio [RR], 1.72, p < 0.0043) [18]. Unadjusted analyses of risk ratios were similarly significant when comparing risk of mortality (RR 2.28, p = 0.0069), thrombosis (RR 3.55, p < 0.001), and ICU requirement (RR 2.64, p = 0.0013). However, when adjusted analyses were performed, there was no difference in mortality, thrombosis or ICU requirement. Another study found that in 111 LT patients, mycophenolate immunosuppression was an independent predictor of severe COVID-19 (RR 3.94 [95% CI 1.59–9.74], p = 0.003) [19]. In light of this, dose reduction upon hospital admission could potentially curb a severe COVID-19 course, however, complete cessation of immunosuppression is discouraged. Furthermore, due to the strain that COVID-19 has placed on healthcare systems, LT transplant candidates are undergoing higher levels of risk stratification and consideration, in part due to our continuously evolving understanding of donor-derived disease [20]. Waiting times have increased dramatically. While this careful management and scrutiny is necessitated by the current pandemic, the future healthcare toll and burden that these delays will undoubtedly bring about h not clear yet.
As we tread uncharted waters in combating a generation-defining pandemic, it is imperative that we continue to cogitate and devise novel therapeutic strategies for infected patients with preexisting liver comorbidities. It is indeed through a meticulous understanding of the viral pathophysiology that extraintestinal manifestations can be curbed, with a subsequent amelioration of disease outcomes in the aforesaid patient population.
Ethical approval
NA.
Sources of funding
NA.
Author contribution
TA, ME, TK: conceived the idea, designed the study, and drafted the manuscript.
AH, ARN, SH: conducted literature search and created the illustrations.
RA, AHA: revised the manuscript critically and refined the illustrations.
SS, EM: revised the final version of the manuscript critically and gave the final approval.
Consent
NA.
Registration of research studies
-
1.
Name of the registry: NA.
-
2.
Unique Identifying number or registration ID: NA.
-
3.
Hyperlink to your specific registration (must be publicly accessible and will be checked): NA.
Guarantor
Talal Almas.
RCSI University of Medicine and Health Sciences.
123 St. Stephen's Green.
Dublin 2, Ireland.
+353834212442.
Declaration of competing interest
NA.
References
- 1.Behzad S., Aghaghazvini L., Radmard A.R., Gholamrezanezhad A. Extrapulmonary manifestations of COVID-19: radiologic and clinical overview. Clin. Imag. 2020;66:35–41. doi: 10.1016/j.clinimag.2020.05.013. PMID: 32425338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alqahtani S.A., Schattenberg J.M. Liver injury in COVID-19: the current evidence. United Eur. Gastroenterol. J. 2020;8(5):509–519. doi: 10.1177/2050640620924157. PMID: 32450787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nardo A.D., Schneeweiss-Gleixner M., Bakail M., Dixon E.D., Lax S.F., Trauner M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2020;41(1) doi: 10.1111/liv.14730. published online Nov 20, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu J., Song S., Cao H.C., Li L.J. Liver diseases in COVID-19: etiology, treatment and prognosis. World J. Gastroenterol. 2020;26(19):2286–2293. doi: 10.3748/wjg.v26.i19.2286. PMID: 32476793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chai X., Hu L., Zhang Y. 2020. Specific ACE2 Expression in Cholangiocytes may Cause Liver Damage after 2019-nCoV Infection. bioRxiv. [DOI] [Google Scholar]
- 6.Yang L., Han Y., Nilsson-Payant B.E. A human pluripotent stem cell-based platform to study SARS-CoV-2 tropism and model virus infection in human cells and organoids. Cell Stem Cell. 2020;27:125–136. doi: 10.1016/j.stem.2020.06.015. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Téllez L., Martín Mateos R.M. COVID-19 and liver disease: an update. Gastroenterol. Hepatol. 2020;43(8):472–480. doi: 10.1016/j.gastrohep.2020.06.006. PMID: 32727662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu L., Liu J., Lu M., Yang D., Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40(5):998–1004. doi: 10.1111/liv.14435. PMID: 32170806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ridruejo E., Soza A. The liver in times of COVID-19: what hepatologists should know. Ann. Hepatol. 2020;19(4):353–358. doi: 10.1016/j.aohep.2020.05.001. PMID: 32425991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang C., Shi L., Wang F.S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol. Hepatol. 2020;5(5):428–430. doi: 10.1016/S2468-1253(20)30057-1. PMID: 32145190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan W.-J., Ni Z.-Y., Hu Y. Clinical characteristics of 2019 novel coronavirus infection in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. published online Feb 28, 2020. [DOI] [Google Scholar]
- 12.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. J. Am. Med. Assoc. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. Erratum in: JAMA. 2020 May 26;323(20):2098. PMID: 32320003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bangash M.N., Patel J., Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol. Hepatol. 2020;5(6):529–530. doi: 10.1016/S2468-1253(20)30084-4. PMID: 32203680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai Q., Huang D., Yu H. COVID-19: abnormal liver function tests. J. Hepatol. 2020;73(3):566–574. doi: 10.1016/j.jhep.2020.04.006. PMID: 32298767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurokawa T., Ohkohchi N. Chapter 9 - role of platelet, blood stem cell, and thrombopoietin in liver regeneration, liver cirrhosis, and liver diseases. In: Yun-Wen Zheng, editor. Stem Cells and Cancer in Hepatology. Academic Press; 2018. pp. 159–177. [DOI] [Google Scholar]
- 16.Kovalic A.J., Satapathy S.K., Thuluvath P.J. Prevalence of chronic liver disease in patients with COVID-19 and their clinical outcomes: a systematic review and meta-analysis. Hepatol. Int. 2020;14(5):612–620. doi: 10.1007/s12072-020-10078-2. PMID: 32725453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo X.M., Zhou W., Xia H., Yang W., Yan X., Wang B., Guo T. Characteristics of SARS-CoV-2 infected patients with clinical outcome during epidemic ongoing outbreak in Wuhan, China. SSRN Electron. J. 2020;12 [Google Scholar]
- 18.Mansoor E., Perez A., Abou-Saleh M. Clinical characteristics, hospitalization, and mortality rates of coronavirus disease 2019 among liver transplant patients in the United States: a multicenter research network study. Gastroenterology. 2021;160(1):459–462. doi: 10.1053/j.gastro.2020.09.033. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colmenero J., Rodríguez-Perálvarez M., Salcedo M. Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J. Hepatol. 2021;74(1):148–155. doi: 10.1016/j.jhep.2020.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fix O.K., Hameed B., Fontana R.J. Clinical best practice advice for hepatology and liver transplant providers during the COVID‐19 pandemic: AASLD expert panel consensus statement. Hepatology. 2020;72(1) doi: 10.1002/hep.31281. [DOI] [PMC free article] [PubMed] [Google Scholar]