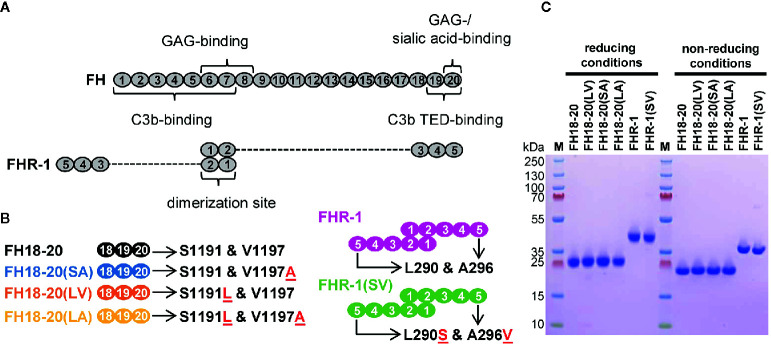
Figure 1.
Schematic structure and sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis of the recombinantly expressed FH18-20 and FHR-1 variants used in this study. (A) Schematic structure of FH and FHR-1. Every oval represents a CCP domain. The first two CCP domains of FHR-1 (dimerization site) share 42 and 34% identity with the corresponding CCPs in FH (CCPs 6 and 7). FHR-1 domains 3, 4, and 5 share 100, 100, and 98% identity with CCPs 18, 19, and 20 in FH. Previously reported binding sites are shown. (B) Schematic representation of the proteins used in this study. Mutations are indicated by the red underlined one letter code amino acid found in the mutant. The name of each construct, except for the wild type species, reports in brackets the amino acids corresponding to positions 1,191 and 1,197 (for FH) or 290 and 296 (for FHR-1). For clarity, the color used to depict each construct is maintained throughout the text and figures. (C) SDS-PAGE analysis of recombinantly expressed FH18-20 and FHR-1 variants. 2 μg of each protein were loaded under both reducing and non-reducing conditions on a 4-12% Bis-Tris gradient gel, then stained with Coomassie Brilliant Blue R250.