Figure 5.
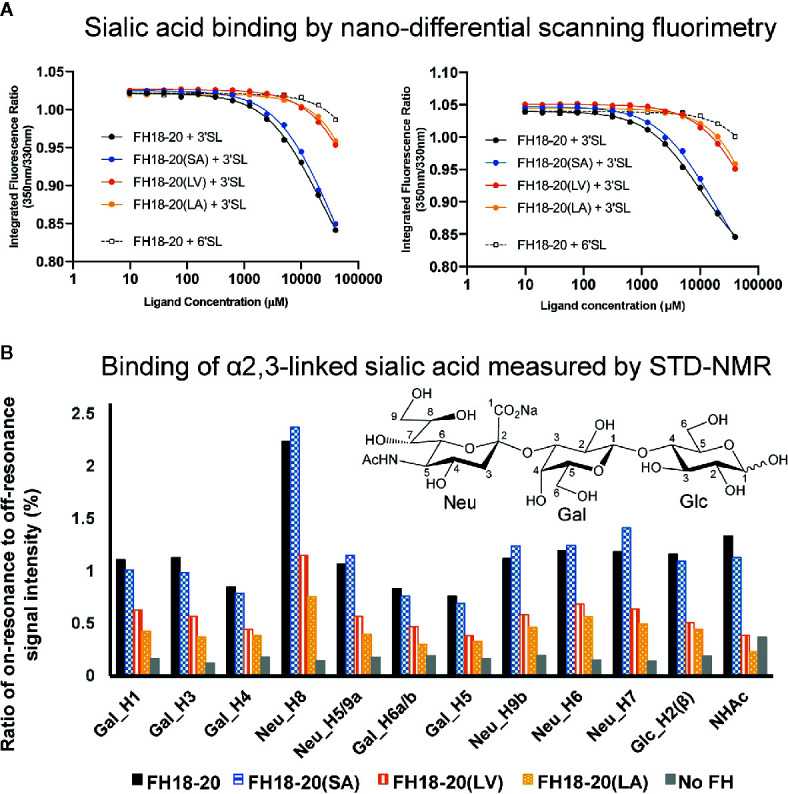
Assessment of the interaction between monomeric FH18-20 constructs and sialyllactose in solution. (A) Nano-differential scanning fluorimetry (nano-DSF) measurements. Determination of the intrinsic tryptophan fluorescence of wildtype, single, and double mutants of FH18-20 in the presence of either α2,3-linked sialic acid (3’SL) or α2,6-linked sialic acid (6’SL) was performed at 20°C (left panel) and 37°C (right panel). The different FH18-20 constructs (25 µM) were incubated with increasing amounts of sialylated ligand (9.8 µM–40 mM), and then excited at 285 nm and the fluorescence emission recorded at 330 and 350 nm. Concentrations were measured in triplicate and the full experiment run in duplicate to ensure reproducibility. (B) α2,3-Linked sialic acid affinity measurements by STD-NMR. α2,3-Linked sialic acid binding of FH18-20 and its mutational variants was determined by STD-NMR. The histograms report the ratio of on-resonance to off-resonance signal intensities (%) measured upon binding of FH18-20 constructs to different hydrogen atoms (H) of α2,3-linked sialyllactose. The order of signals along the x-axis reflects their relative chemical shifts in the NMR spectra. A chemical structure for α2,3-linked sialyllactose is provided for reference.
