Highlights
-
•
TB progressed in 58.4 % (191/327). More than 90 % of participants with TBI = 2 or 3 (51/56) had TB progression.
-
•
TB progression was associated with increased mortality (adjusted HR = 1.68, P < 0.001).
-
•
Median overall survival (OS) were 11.56 years for TB progression group and 13.92 years for No TB progression group (P < 0.001), respectively.
Abbreviations: AGES-Reykjavik Study, Age Gene/Environment Susceptibility-Reykjavik Study; BMI, body mass index; HR, hazard ratio; ILA, interstitial lung abnormalities; ILD, interstitial lung disease; OS, overall survival; TB, traction bronchiectasis; TBI, traction bronchiectasis/bronchiolecetasis index; TBI-R2, traction bronchiectasis/bronchiolecetasis index on Round 2
Keywords: Interstitial lung abnormality, Usual interstitial pneumonia, Pulmonary fibrosis, Traction bronchiectasis, Age Gene/Environment Susceptibility-Reykjavik Study
Abstract
Purpose
The aim of this study is to assess the role of traction bronchiectasis/bronchiolectasis and its progression as a predictor for early fibrosis in interstitial lung abnormalities (ILA).
Methods
Three hundred twenty-seven ILA participants out of 5764 in the Age, Gene/Environment Susceptibility (AGES)-Reykjavik Study who had undergone chest CT twice with an interval of approximately five-years were enrolled in this study. Traction bronchiectasis/bronchiolectasis index (TBI) was classified on a four-point scale: 0, ILA without traction bronchiectasis/bronchiolectasis; 1, ILA with bronchiolectasis but without bronchiectasis or architectural distortion; 2, ILA with mild to moderate traction bronchiectasis; 3, ILA and severe traction bronchiectasis and/or honeycombing. Traction bronchiectasis (TB) progression was classified on a five-point scale: 1, Improved; 2, Probably improved; 3, No change; 4, Probably progressed; 5, Progressed. Overall survival (OS) among participants with different TB Progression Score and between the TB progression group and No TB progression group was also investigated. Hazard radio (HR) was estimated with Cox proportional hazards model.
Results
The higher the TBI at baseline, the higher TB Progression Score (P < 0.001). All five participants with TBI = 3 at baseline progressed; 46 (90 %) of 51 participants with TBI = 2 progressed. TB progression was also associated with shorter OS with statistically significant difference (adjusted HR = 1.68, P < 0.001).
Conclusion
TB progression was visualized on chest CT frequently and clearly. It has the potential to be the predictor for poorer prognosis of ILA.
1. Introduction
Traction bronchiectasis or bronchiolectasis is one of the CT features of usual interstitial pneumonia [1], representing pathologic dilatation of bronchi or bronchiole, which results from the retractile fibrotic process according to the areas of pulmonary fibrosis or architectural distortion and leads to the formation of honeycombing [[1], [2], [3]]. It is sometimes difficult to distinguish traction bronchiectasis from microscopic honeycombing [4]. According to several radiological studies, traction bronchiectasis can be associated with mortality or poor outcome in interstitial lung disease (ILD) [[5], [6], [7], [8]]. Interstitial lung abnormalities (ILA) are incidentally detected on chest CT without clinical symptoms, found in 3–17 % of cases in large cohort studies [[9], [10], [11], [12], [13]]. Imaging findings of ILA include nondependent ground-glass or reticular abnormalities, diffuse centrilobular nodularity, nonemphysematous cysts, honeycombing, and traction bronchiectasis [[9], [10], [11], [12],[14], [15], [16], [17]]. Multiple studies have reported that a proportion of subjects with ILA progress to the early phase of ILD [9,10,[14], [15], [16], [17]]. Some reports showed that ILA and its progression are associated with decreased pulmonary function or increased mortality [14,15,[18], [19], [20], [21], [22], [23]].
It is not known whether traction bronchiectasis progresses or improves over time, although many pulmonologists, radiologists, and pathologists believe that traction bronchiectasis is probably irreversible and progressive. It is also unknown whether progression of traction bronchiectasis/bronchiolectasis is associated with overall survival (OS).
The aims of this study are to answer the following fundamental questions: (1) Does traction bronchiectasis progress or improve over time on observational study on CT scans? (2) Are there associations between progression of bronchiectasis/bronchiolectasis and OS? The overarching hypothesis of this study is that traction bronchiectasis and bronchiolectasis may serve as a simpler and more reliable sign of early fibrosis in ILA.
2. Materials and methods
2.1. Study population
This study was approved by the National Bioethics Committee in Iceland (VSN: 00-063) and the institutional review boards of Brigham and Women’s Hospital. Written informed consents were obtained from all the participants. Protocol of participant enrollment was same as previous studies had reported [18,23]. The Age, Gene/Environment Susceptibility (AGES)-Reykjavik Study is a longitudinal birth cohort derived from the Reykjavik Study, which was established in 1967 and includes men and women that were born in Reykjavik, Iceland from 1907 to 1935 and are now followed by the Icelandic Heart Association [23]. Previous studies have examined the association between mortality and ILA assessed by chest CT [18,24]. Recently, we have also reported the association between traction bronchiectasis/brochiolectasis and ILA mortality in 378 participants with ILA at baseline [8]. Of the 5764 participants with baseline CT scans recruited from 2002 to 2006, 3167 participants had follow-up/Round 2 chest CT scans from 2007 to 2011 (approximately five years after baseline CT). Time-to-mortality was ascertained at the end of 2016 (at least five-year interval after Round 2 CT scan). All the CT images had been assessed and the presence or absence of both ILA and its progression had been identified in the previous study [24]. For this study, we evaluated 327 subjects who underwent follow-up chest CT and were diagnosed as ILA with chest CT either on baseline or Round 2 CT scans, or both (Fig. 1). Demographic data such as age, body mass index (BMI), sex, and smoking as well as time-to-mortality were collected.
Fig. 1.
Flow charts of inclusion and exclusion criteria for this study.
2.2. Traction bronchiectasis/bronchiolectasis index (TBI) and its progression on CT images
Traction bronchiectasis/bronchiolectasis was defined as dilatation of airway (i.e. bronchi and bronchioles) within areas demonstrating ILA on chest CT. In 327 subjects with ILA, we scored the severity of traction bronchiectasis/bronchiolectasis index (TBI) using a categorical 4-point score as previously reported [8]: 0, ILA without traction bronchiectasis/bronchiolectasis; 1, ILA with bronchiolectasis but without bronchiectasis or architectural distortion; 2, ILA with mild to moderate traction bronchiectasis; 3, ILA and severe traction bronchiectasis and/or honeycombing. Progression of traction bronchiectasis (TB) was evaluated with a categorical 5-point score: 1, Improved; 2, Probably improved; 3, No change; 4, Probably progressed; 5, Progressed (Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7).
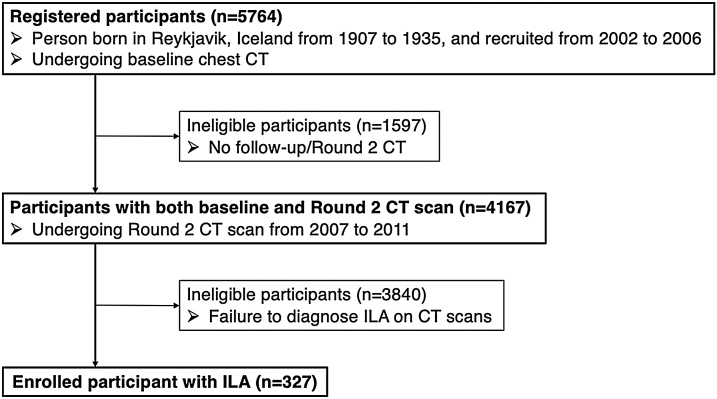
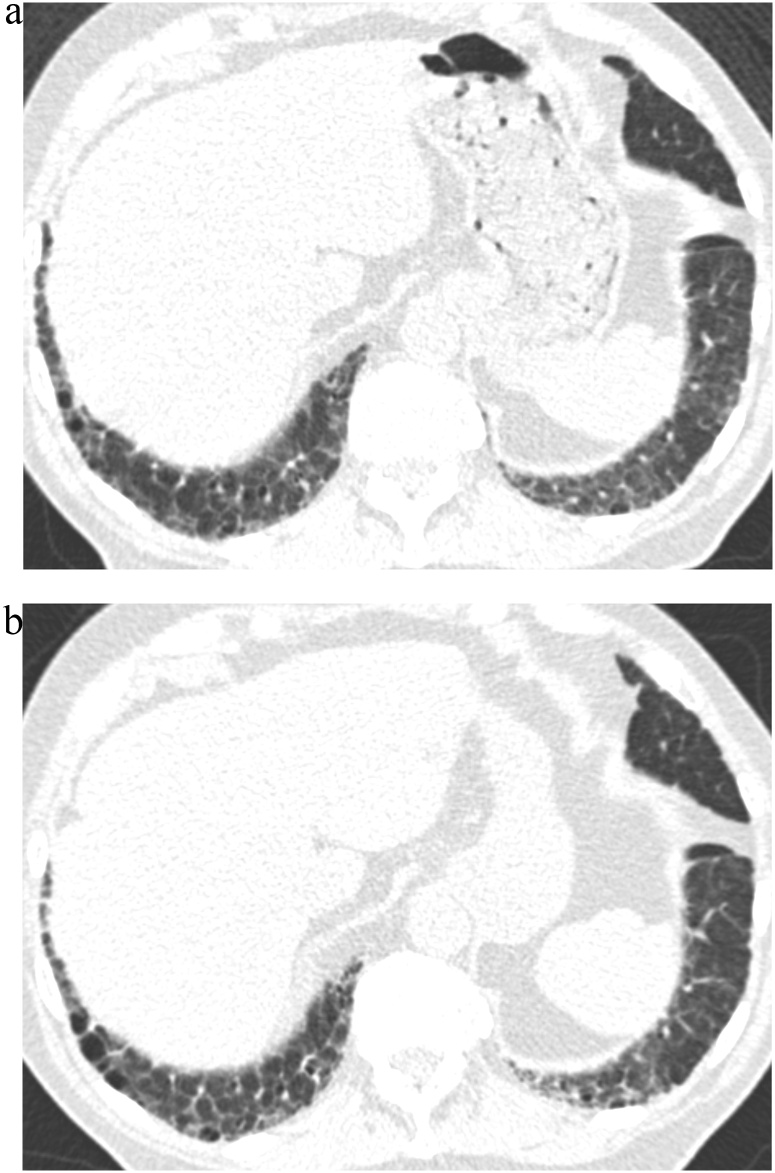
Fig. 2.
TB Progression Score 5.
(a) TBI = 2. CT image demonstrated ground-glass and reticular opacities with sub-pleural and basal distribution with mild traction bronchiectasis indicating Subpleural fibrotic ILA.
(b) TBI = 3. Follow-up CT image demonstrated marked interval progression of ground-glass and reticular opacities with sub-pleural and basal distribution. Note is made of severe traction bronchiectasis associated with architectural distortion.
TBI, traction bronchiectasis/bronchiolectasis index; ILA, interstitial lung abnormality
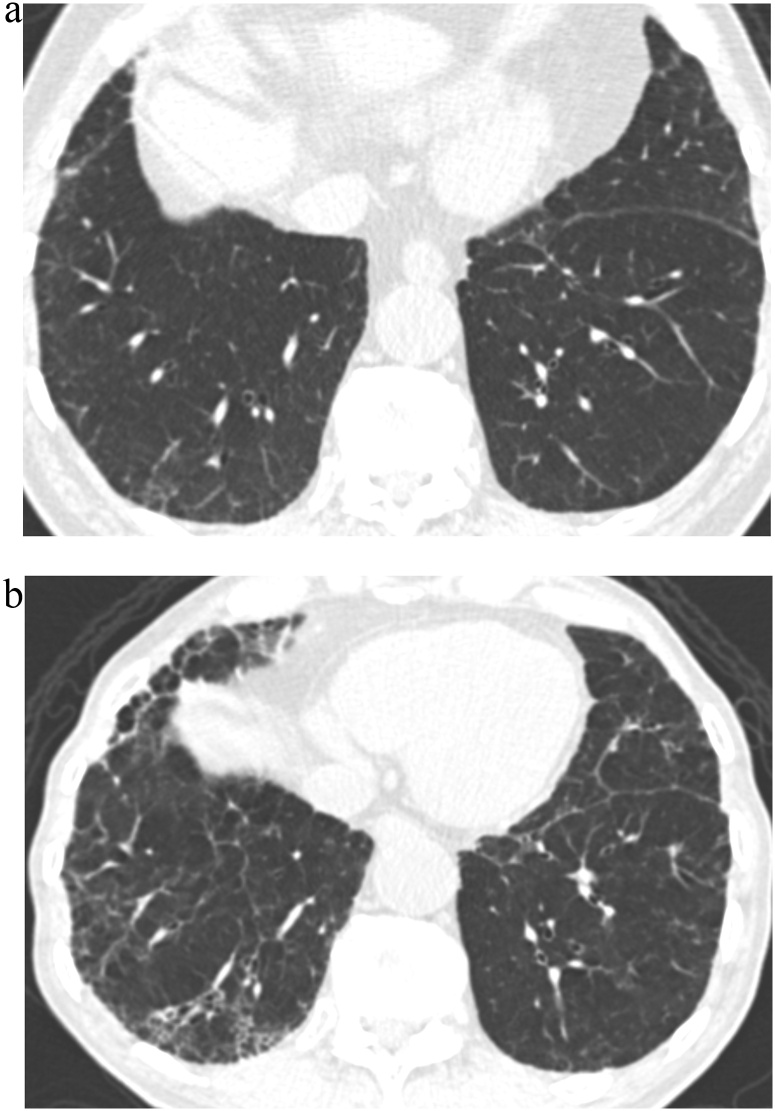
Fig. 3.
TB Progression Score 5.
(a) TBI = 3. CT image demonstrated ground-glass and reticular opacities with sub-pleural and basal predominance associated with severe traction bronchiectasis and architectural distortion.
(b) TBI = 3. Follow-up CT image demonstrated further progression of fibrotic lung disease with severe traction bronchiectasis and honeycombing.
TBI, traction bronchiectasis/bronchiolectasis index
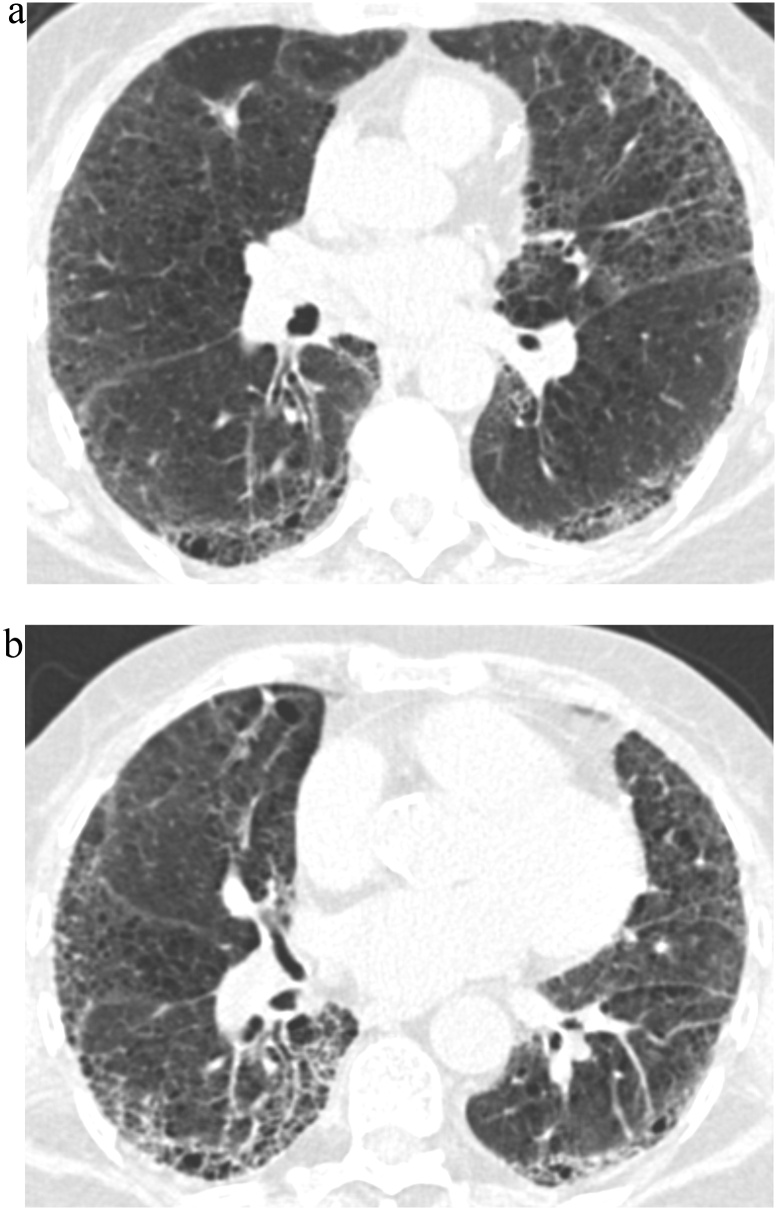
Fig. 4.
TB Progression Score 5.
(a) TBI = 2. CT image demonstrated ground-glass and reticular opacities with sub-pleural and basal distribution and mild traction bronchiectasis associated with architectural distortion in the left basal area.
(b) TBI = 2. Follow-up CT image demonstrated obvious progression of ground-glass and reticular opacities with moderate traction bronchiectasis and increased architectural distortion.
TBI, traction bronchiectasis/bronchiolectasis index
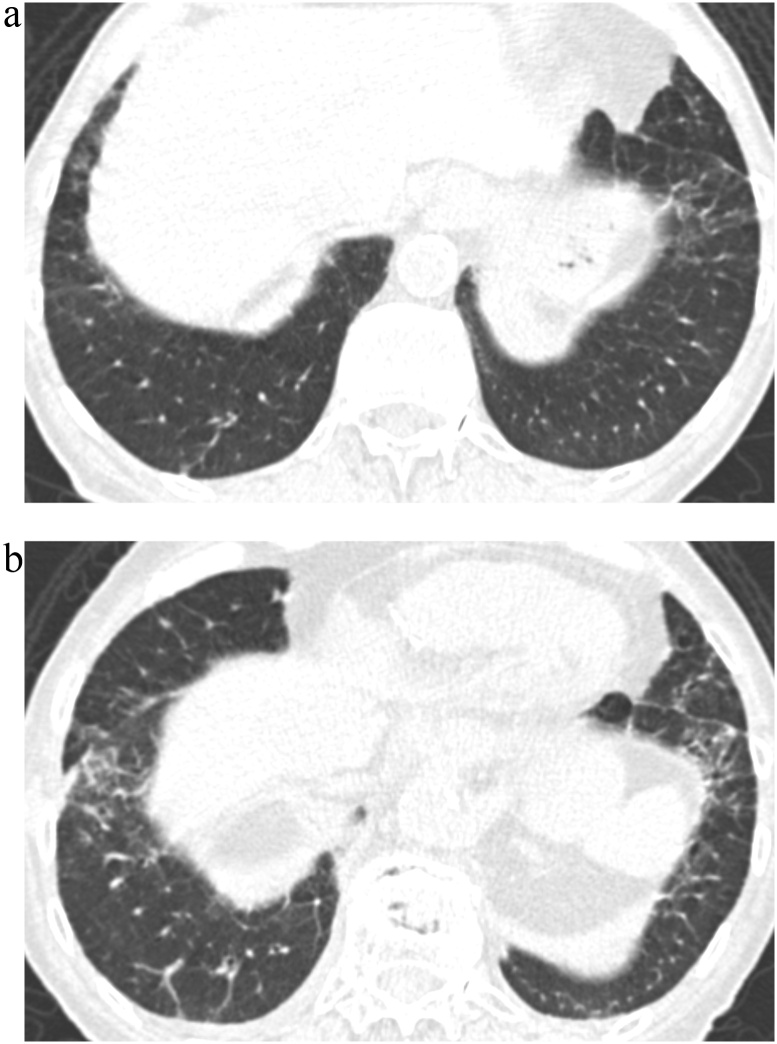
Fig. 5.
TB Progression Score 4.
(a) TBI = 2. CT image demonstrated ground-glass reticular opacities with sub-pleural and basal predominance with mild traction bronchiectasis associated with architectural distortion.
(b) TBI = 2. Follow-up CT image demonstrated probable increase in traction bronchiectasis and architectural distortion.
TBI, traction bronchiectasis/bronchiolectasis index
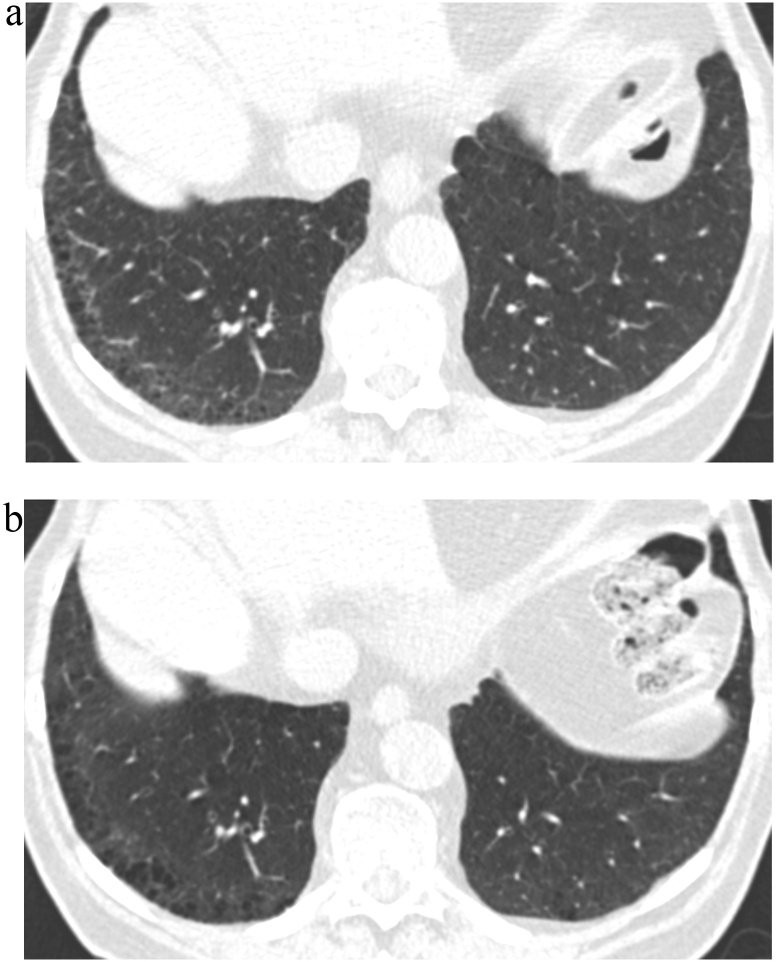
Fig. 6.
TB Progression Score 2.
(a) TBI = 1. CT image demonstrated ground-glass and reticular opacities with sub-pleural and basal distribution.
(b) TBI = 1. Follow-up CT image demonstrated probable dedrease in ground-glass and reticular opacities in the interval.
TBI, traction bronchiectasis/bronchiolectasis index
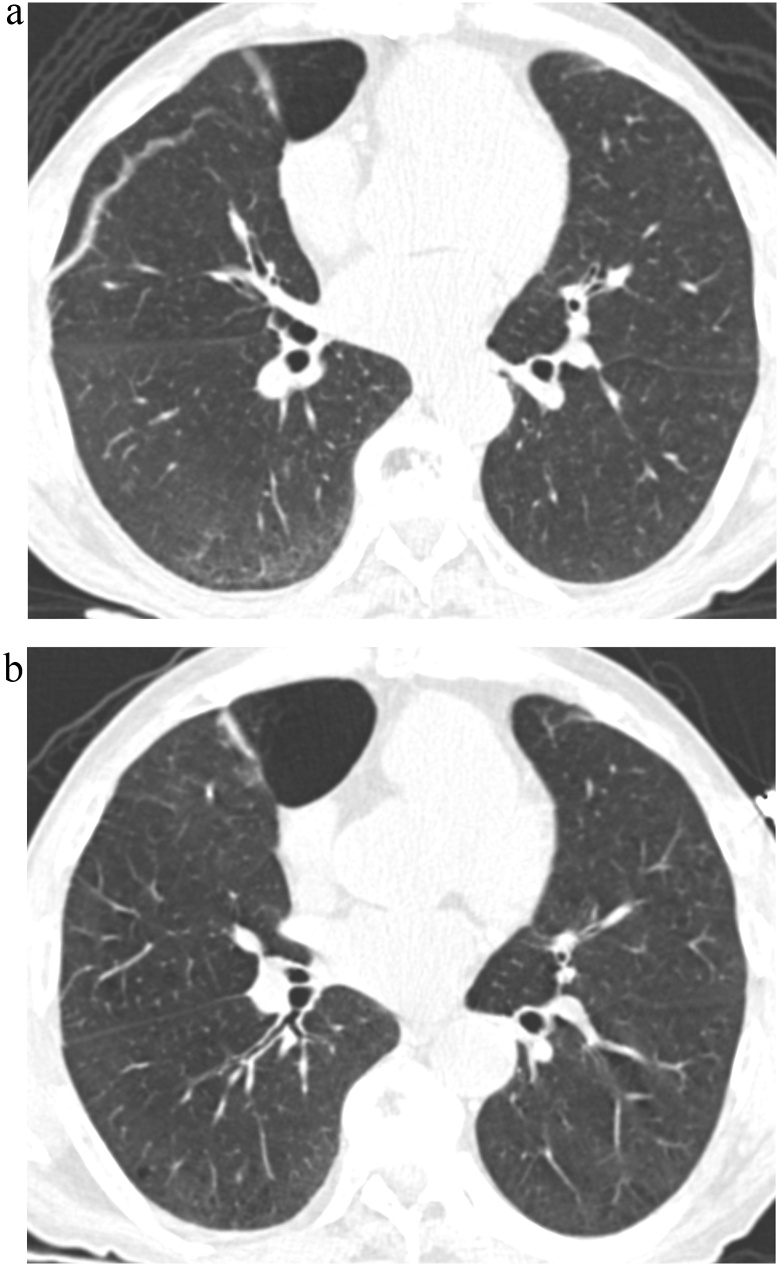
Fig. 7.
TB Progression Score 1.
(a) TBI = 1. CT image demonstrated ground-glass and reticular opacities with sub-pleural and basal distribution associated with bronchilectasis.
(b) TBI = 0. Follow-up CT image demonstrated the near-complete resolution of the previously identified sub-pleural ground-glass and reticular opacities as well as bronchiolectgasis.
TBI, traction bronchiectasis/bronchiolectasis index
Two experienced thoracic radiologists (T.H and H.H) interpreted the anonymized images of chest CT and scored TBI of both baseline (TBI) and follow-up/Round 2 (TBI-R2) by consensus, using workstation (Vitrea; Canon Medical Systems Corp., Ohtawara, Japan). TB Progression Score was determined by observing CT images of both rounds as a consensus. TB progression group was defined as TB Progression Score equal to 4 or 5; No TB progression group was defined as TB Progression Score equal to 1 or 2 or 3. The image analysis data were sent to Iceland Heart Association after the image review, and the data including survival were then provided to the investigator’s team after deidentification, so that the personal data of each participant were not identifiable. The demographic and clinical data of all the participants were blinded to the two radiologists.
2.3. Statistical analysis
Demographic data, except for sex and smoking history, was examined with one-way analysis of variance. The difference of distribution of sex or smoking history among groups was studied with Chi-squared test. The overall survival (OS) was assessed with Kaplan-Meier estimator. Survival curves among the subjects with different TB Progression Score or TBI-R2 were examined with the correction of Holm-Bonferroni method. OS was defined as the particular period from the recruitment to the AGES-Reykjavik study to the date of death of any cause or the end of observation. Hazard ratio (HR) of TB progression group was calculated with Cox proportional hazards model adjusted with age, BMI, sex, smoking history, and pack-year.
Statistical analyses were conducted with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), and graphical user interface for R 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria) [25]. More precisely, it is a modified version of R commander designed to add statistical functions frequently used in biostatistics. A two-sided value of P < 0.05 was considered statistically significant.
3. Results
When the degree of TB was compared (TB Progression Score) among 327 participants studied, 4 were improved (Score 1); 2 were probably improved (Score 2); 130 were unchanged (Score 3); 46 were probably progressed (Score 4); and 145 were progressed (Score 5). Of the 327 participants, 136 (41.6 %) were classified as No TB progression group (Score 1, 2, or 3) and 191 (58.4 %) were classified as TB progression group (Score 4 or 5). The higher the TBI at baseline, the higher the TB Progression Score (P < 0.001) (Table 1). The participants with TBI = 3 (n = 5) at baseline were classified as Progression group in 100 %; participants with TBI = 2 (n = 51) in 90 %; participants with TBI = 1 (n = 65) in 57 %; participants with TBI = 0 (n = 51) in 20 %. Demographic data are shown in Table 2. Demographic data, except for sex, showed no statistically significant difference among different TB Progression groups.
Table 1.
TBI on both first and second round chest CT.
| TB Progression Score / 5-year-interval |
||||||||
|---|---|---|---|---|---|---|---|---|
| Score 1 | Score 2 | Score 3 | Score 4 | Score 5 | ||||
| Improved | Probably Improved | No change | Probably Progressed | Progressed | ||||
| No TB progression group |
TB progression group |
|||||||
| TBI | n | (n = 4) | (n = 2) | (n = 130) | (n = 46) | (n = 145) | P value | |
| ILA at baseline | TBI = 3 | 5 | 0 | 0 | 0 | 0 | 5 | <0.001† |
| TBI = 2 | 51 | 0 | 0 | 5 | 10 | 36 | ||
| TBI = 1 | 65 | 4 | 2 | 22 | 24 | 13 | ||
| TBI = 0 | 51 | 0 | 0 | 41 | 2 | 8 | ||
| No ILA at baseline | 155 | 0 | 0 | 62 | 10 | 83 | ||
TB, traction bronchiectasis; TBI, traction bronchiectasis/bronchiolectasis index; ILA, interstitial lung abnormalities.
P value is for the comparison among all groups by one-way analysis of variance on ranks.
Table 2.
Demographic data of participants.
| TB Progression Score / 5-year-interval |
||||
|---|---|---|---|---|
| Score 1, 2, 3 | Score 4 | Score 5 | ||
| No change or Improved | Probably Progressed | Progressed | ||
| No TB progression group | TB progression group |
|||
| (n = 136)* | (n = 46) | (n = 145) | P value | |
| Age, years | 75.1 ± 4.5 [67−87] | 76.5 ± 5.5 [68−91] | 76.0 ± 5.2 [67−89] | 0.15† |
| BMI | 27.8 ± 5.2 [17.5−46.6] | 27.3 ± 3.7 [18.6−36.6] | 27.9 ± 3.8 [17.3−45.0] | 0.70† |
| Sex, n | 0.048‡ | |||
| Male | 61 | 27 | 85 | |
| Female | 75 | 19 | 60 | |
| Smoking history, n | 0.052‡ | |||
| Never | 47 | 17 | 31 | |
| Former | 67 | 24 | 95 | |
| Current | 21 | 5 | 19 | |
| Unknown | 1 | 0 | 0 | |
| Pack-year | 7.8 [0−178.6] | 10.0 [0−64.0] | 12.3 [0−116.1] | 0.61† |
TBI; traction bronchiectasis/bronchiolectasis index.
Age and BMI are described as mean ± standard deviation [range], while Pack-year is described as median [range].
TB Progression Score 1 (n = 4) and Score 2 (n = 2) are included in Score 3 (n = 130) due to small number of cases.
P value is for the comparison among all groups by one-way analysis of variance.
P value is for the comparison among all groups by Chi-squared test.
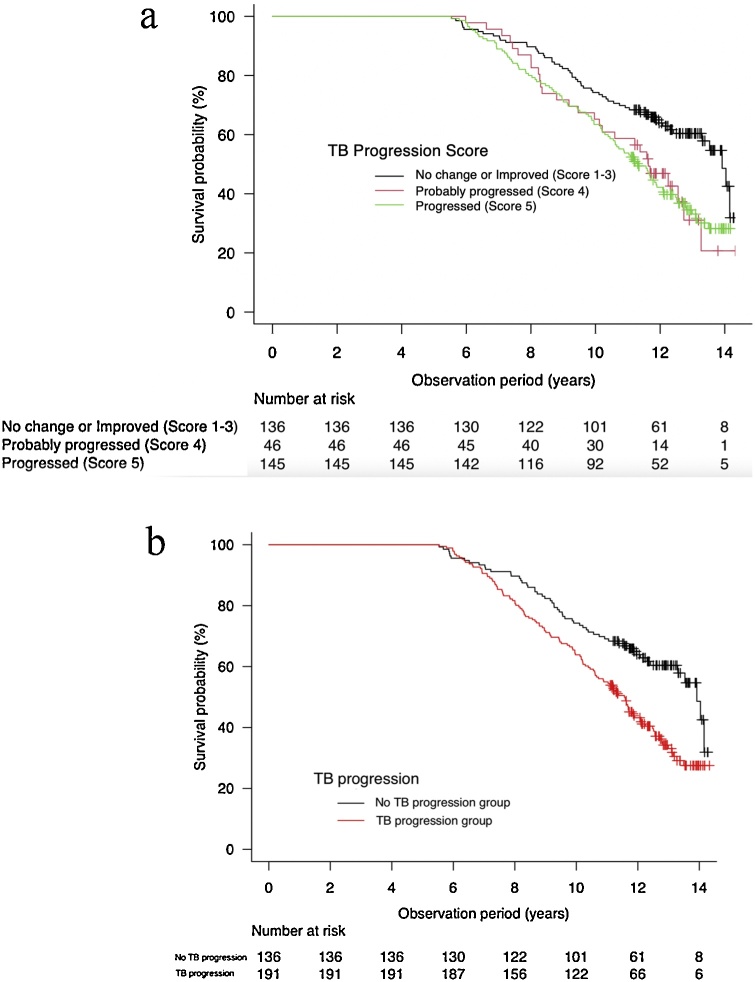
Fig. 7 showed Kaplan-Meier survival curves among participants with different TB Progression Scores (Fig. 8a) and between participants with No TB progression and Progression groups (Fig. 8b). Due to the small number of cases, TB Progression Score 1 (n = 4) and Score 2 (n = 2) was summarized into Score 3 (n = 130) as No TB progression group. Statistically significant difference on survival curves were observed between No TB progression group and TB Progression Score 4 (Probably Progressed) (P = 0.022), and between No TB progression group and Score 5 (Progressed) (p < 0.001). The Cox proportional hazard model indicated that TB progression is associated with increased all-cause mortality in the long-term observation period (adjusted with age, sex, BMI, smoking history and pack-year: HR = 1.68; 95 %CI = 1.21–2.34, P < 0.001). The median OS were 11.56 years (95 %CI; 10.62–12.08) for Progression group and 13.92 years (95 %CI; 13.30 – not reached) for No TB progression group.
Fig. 8.
(a) Survival curves among participants stratified by TB Progression Score. TB Progression Score 1 (n = 4) and Score 2 (n = 2) are included in Score 3 due to small number of cases. The survival curve of Score 3 was different with statistical significance from that of Score 4 and Score 5 (P = 0.022, P < 0.001, respectively).
(b) Survival curves among participants stratified by the presence of TB progression. TB progression group increased the risk for all-cause mortality (adjusted HR = 1.68; 95 %CI = 1.21–2.34, P < 0.001). Median OS were 11.56 years (95 %CI; 10.62–12.08) for TB progression group and 13.92 years (95 %CI; 13.30 – not reached) for No TB progression group, respectively.
TB, traction bronchiectasis; OS, overall survival; HR, hazard ratio
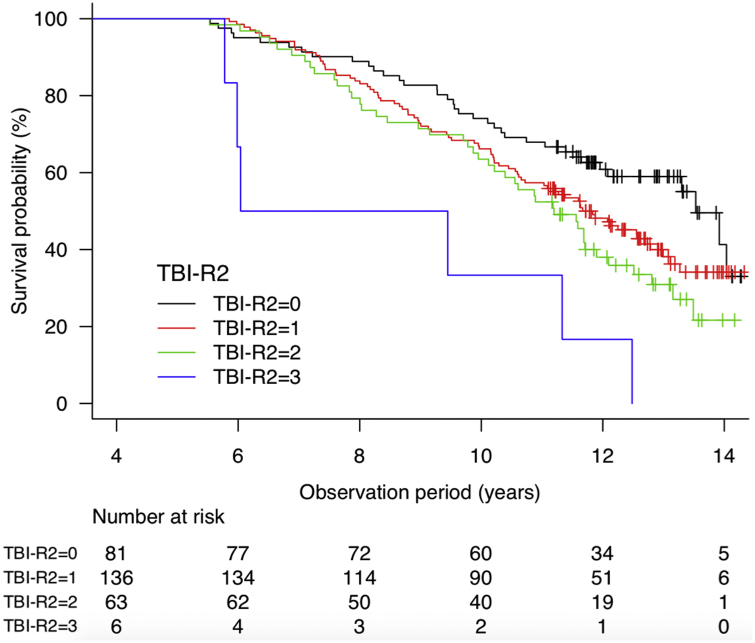
The data on TBI-R2 and Kaplan-Meier survival curve based upon TBI-R2 are provided as Supplement with Table E1 and Fig. E1.
4. Discussion
This report confirmatory proved radiographic progression of traction bronchiectasis over approximately 5 years. TB progression was observed in participants with higher TBI at baseline. In addition, TB progression is associated with increased risks for death.
Since there was no significant difference in age, BMI, smoking history or pack-year among different TB progression groups, except for a trend of higher TB progression score in male participants, TB probably progresses regardless of age, and smoking. It was not surprising to find that TB progressed in 90–100 % of participants with mild, moderate and severe TB at baseline (TBI = 2 or 3). No participants with TB at baseline demonstrated the improvement of TB in five-year interval, which confirmed the assumption that TB is an irreversible radiographic and pathological process characterizing pulmonary fibrosis. On the other hand, participants with traction bronchiolectasis (TBI = 1; n = 65) demonstrated improvement in 6 (9%), no change in 22 (34 %), and progression in 37 (57 %). This result suggests that traction bronchiolectasis may be a marker of early pulmonary fibrosis. Conversely, lack of traction bronchiolectasis in ILA participants (TBI = 0) appears to serve as benign prognostic marker, considering only 20 % showed interval development of TB/traction bronchiolectasis.
The prognostic importance of TB/traction bronchiolectasis is in keeping with previous studies which have reported that the degree of TB was an influential factor for the prognosis of ILA, idiopathic pulmonary fibrosis and chronic hypersensitivity pneumonitis [5,6,8,26]. Approximately half of the participants in TB progression group (93/191) had no ILA at baseline, indicating the development of ILA in the interval of five years. Alternatively, some of these cases may be underrecognized at the baseline evaluation of ILA. Further investigation of this group is required.
Kaplan-Meier survival curve demonstrated the association of TB progression with mortality (P < 0.001). The survival curve showed that TB progression group has increased risk of all-cause mortality compared with No TB progression group (adjusted HR = 1.68; 95 %CI = 1.21–2.34, P < 0.001). Recently, Jacob et al. [27] have reported that interval change of severity of TB was the most influential factor of mortality in IPF patients despite declines of forced vital capacity. Suggestively, TB has the potential to predict OS with independence of background pulmonary fibrosis or pulmonary function. We note that 155 subjects (47,4%) out of 327 subjects did not have ILA at the baseline as shown in Table 1. When OS was compared between 155 subjects with ILA and 172 subjects without ILA, no statistical difference in OS was observed (p = 0.409). The concept of the spectrum from ILA to UIP was emerging and getting attraction [15]. Fibrotic ILA, often accompanying TB/traction bronchieolctasis, and its temporal change may be more noteworthy than ever.
TB on chest CT has been observed as pathological honeycombing or cysts around peripheral airway [15,28,29]. Peripheral TB on HRCT was emphasized on the clinical guideline; possible UIP pattern, which sometimes resembled ILA, could correspond to pathological UIP under the presence of TB on HRCT [10,13,30]. On the other hand, pathological honeycombing or TB were sometimes observed in subjects with ILA without TB on chest CT [15,28,31]. In several studies, honeycombing could be considered a result of remodeling from TB [28,[32], [33], [34]]. Staats et al. [28] have reported that the degree of honeycombing on high-resolution CT was significantly correlated with that of both pathological traction bronchiolectasis and dilated cysts around pulmonary epithelium or fibroblast foci. In terms of both pathology and radiology, TB/traction bronchiolectasis and honeycombing are considered to be associated with each other in the continuous spectrum of bronchiolar remodeling.
Our study has several limitations. First, the age of participants in this cohort is relatively high due to the characteristics of the AGES-Reykjavik study. It includes men and women born from 1907 to 1935; the average age of participants enrolled in this study amounted to seventy-six. Hoyer et al. [35] reported that ILA subjects were subject to respiratory disease such as chronic obstructive pulmonary disease, lung cancer and pneumonia. However, the cause of death of participants was not identified in our study. Second, the distribution of the enrolled participants differed between the TB progression group and the No TB progression group, with a higher proportion of males in the progression group. However, the survival effect persisted after adjustment for sex. Third, our study depended on only visual evaluation. In the previous study, the visual interobserver agreement of TB was equal to 0.75: substantial agreement [8].
In conclusion, TB progression is associated with higher baseline TBI and shorter OS in ILA patients. The interval change of traction bronchiectasis/bronchiolectasis has the potential to be the predictor for poorer prognosis of ILA.
Guarantor
As the corresponding author, Dr. Takuya Hino declared the responsibility of content of the manuscript, including the data and analysis.
Author contribution
HT, HH contributed to the study design, interpretation, data analysis and the writing of the initial manuscript. JL takes responsibility for the accuracy of data analysis. GEF, GV, GG, and VVI had full access to all of the data in the study and takes responsibility for the integrity of the data. HT, MN, PRK, HA, AT, HO, YM, YY, KT, JM, TN, IK, HH, ERSJ, WGR, JT, CDC, LDA, and HGM contributed substantially to the interpretation and the edit of the manuscript.
Ethical statement
This study was approved by the National Bioethics Committee in Iceland (VSN: 00-063) and the institutional review boards of Brigham and Women’s Hospital. Written informed consents were obtained from all the participants.
The image analysis data were sent to Iceland Heart Association after the image review, and the data including survival were then provided to the investigator’s team after deidentification, so that the personal data of each participant were not identifiable.
This study does not include animal experiment. We use only demographic and image data.
Thus, we state that this study is based on the declaration of Helsinki.
Grant Support (Hatabu)
NIHR01CA203636, 5U01CA209414-03.
NIH/NHLBI2R01HL111024-06.
NIHR01HL135142.
NIH/NHLBI1R01HL130974.
Declaration of Competing Interest
Dr. Nishino reports personal fees from Daiichi Sankyo, from AstraZeneca, grants from Merck investigator studies program, grants from Canon Medical Systems, grants from AstraZeneca, grants from Daiichi Sankyo, personal fees from Roche, outside the submitted work.
Dr. San Jose Estepar reports personal fees from LeukoLabs, grants and personal fees from Boehringer Ingelheim, personal fees from Chiesi, outside the submitted work; and he is also a founder and equity holder of Quantitative Imaging Solutions which is a company that provides image-based consulting and develops software to enable data sharing.
Dr. Hunninghake reports personal fees from Boehringer-Ingelheim, personal fees from Gerson Lehrman Group, personal fees from Mitsubishi Chemical, outside the submitted work.
Dr. Hatabu reports grants from Konica-Minolta Inc, grants from Canon Medical Systems Inc, other from Canon Medical Systems Inc, personal fees from Mitsubishi Chemical Inc, outside the submitted work.
The other authors have no conflicts of interest to be disclosed related to this article.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ejro.2021.100334.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Hansell D.M., Bankier A.A., MacMahon H., McLoud T.C., Müller N.L., Remy J. Fleischner society: glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 2.Westcott J.L., Cole S.R. Traction bronchiectasis in end-stage pulmonary fibrosis. Radiology. 1986;161:665–669. doi: 10.1148/radiology.161.3.3786716. [DOI] [PubMed] [Google Scholar]
- 3.Milliron B., Henry T.S., Veeraraghavan S., Little B.P. Bronchiectasis: mechanisms and imaging clues of associated common and uncommon diseases. Radiographics. 2015;35:1011–1030. doi: 10.1148/rg.2015140214. [DOI] [PubMed] [Google Scholar]
- 4.Zaizen Y., Fukuoka J. Pathology of idiopathic interstitial pneumonias. Surg. Pathol. Clin. 2020;13:91–118. doi: 10.1016/j.path.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Edey A.J., Devaraj A.A., Barker R.P., Nicholson A.G., Wells A.U., Hansell D.M. Fibrotic idiopathic interstitial pneumonias: HRCT findings that predict mortality. Eur. Radiol. 2011;21:1586–1593. doi: 10.1007/s00330-011-2098-2. [DOI] [PubMed] [Google Scholar]
- 6.Sumikawa H., Johkoh T., Colby T.V., Ichikado K., Suga M., Taniguchi H., Kondoh Y., Ogura T., Arakawa H., Fujimoto K., Inoue A., Mihara N., Honda O., Tomiyama N., Nakamura H., Müller N.L. Computed tomography findings in pathological usual interstitial pneumonia. Am. J. Respir. Crit. Care Med. 2008;177:433–439. doi: 10.1164/rccm.200611-1696OC. [DOI] [PubMed] [Google Scholar]
- 7.Jacob J., Bartholmai B.J., Rajagopalan S., Kokosi M., Nair A., Karwoski R., Walsh S.L.F., Wells A.U., Hansell D.M. Mortality prediction in idiopathic pulmonary fibrosis: evaluation of computer-based CT analysis with conventional severity measures. Eur. Respir. J. 2017;49:1601011. doi: 10.1183/13993003.01011-2016. [DOI] [PubMed] [Google Scholar]
- 8.Hida T., Nishino M., Hino T., Lu J., Putman R.K., Gudmundsson E.F., Araki T., Valtchinov V.I., Honda O., Yanagawa M., Yamada Y., Hata A., Jinzaki M., Tomiyama N., Honda H., Estepar R.S.J., Washko G.R., Johkoh T., Christiani D.C., Lynch D.A., Gudnason V., Gudmundsson G., Hunninghake G.M., Hatabu H. Traction bronchiectasis/bronchiolectasis is associated with interstitial lung abnormality mortality. Eur. J. Radiol. 2020;129:109073. doi: 10.1016/j.ejrad.2020.109073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatabu H., Hunnighake G.M., Lynch D.A. Interstitial lung abnormality: recognition and perspectives. Radiology. 2019;291:1–3. doi: 10.1148/radiol.2018181684. [DOI] [PubMed] [Google Scholar]
- 10.Hatabu H., Hunninghake G.M., Richeldi L., Brown K.K., Wells A.U., Remy-Jardin M., Verschakelen J., Nicholson A.G., Beasley M.B., Christiani D.C., San José Estépar R., Seo J.B., Johkoh T., Sverzellati N., Ryerson C.J., Graham Barr R., Goo J.M., Austin J.H.M., Powell C.A., Lee K.S., Inoue Y., Lynch D.A. Interstitial lung abnormalities detected incidentally on CT: a position paper from the Fleischner Society. Lancet Respir. Med. 2020;8:726–737. doi: 10.1016/S2213-2600(20)30168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin G.Y., Lynch D., Chawla A., Garg K., Tammemagi M.C., Sahin H., Misumi S., Kwon K.S. Interstitial lung abnormalities in a CT lung cancer screening population: prevalence and progression rate. Radiology. 2013;268:563–571. doi: 10.1148/radiol.13120816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunninghake G.M., Hatabu H., Okajima Y., Gao W., Dupuis J., Latourelle J.C., Nishino M., Araki T., Zazueta O.E., Kurugol S., Ross J.C., San José R., Estépar E., Murphy M.P.Steele, Loyd J.E., Schwarz M.I., Fingerlin T.E., Rosas I.O., Washko G.R., O’Connor G.T., Schwartz D.A. MUC5B promoter polymorphism and interstitial lung abnormalities. N. Engl. J. Med. 2013;368:2192–2200. doi: 10.1056/NEJMoa1216076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lynch D.A., Sverzellati N., Travis W.D., Brown K.K., Colby T.V., Galvin J.R., Goldin J.G., Hansell D.M., Inoue Y., Johkoh T., Nicholson A.G., Knight S.L., Raoof S., Richeldi L., Ryerson C.J., Ryu J.H., Wells A.U. Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner Society white paper. Lancet Respir. Med. 2018;6:138–153. doi: 10.1016/S2213-2600(17)30433-2. [DOI] [PubMed] [Google Scholar]
- 14.Washko G.R., Hunninghake G.M., Fernandez I.E., Nishino M., Okajima Y., Yamashiro T., Ross J.C., Estépar R.S.J., Lynch D.A., Brehm J.M., Andriole K.P., Diaz A.A., Khorasani R., D’Aco K., Sciurba F.C., Silverman E.K., Hatabu H., Rosas I.O., Investigators C.O.P.D.Gene. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N. Engl. J. Med. 2011;364:897–906. doi: 10.1056/NEJMoa1007285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hino T., Lee K.S., Han J., Hata A., Ishigami K., Hatabu H. Spectrum of pulmonary fibrosis from interstitial lung abnormality to usual interstitial pneumonia: importance of identification and quantification of traction bronchiectasis in patient management. Korean J. Radiol. 2020;21:e196. doi: 10.3348/kjr.2020.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Washko G.R., Lynch D.A., Matsuoka S. Identification of early interstitial lung disease in smokers from the COPDGene Study. Acad. Radiol. 2010;17(1):48–53. doi: 10.1016/j.acra.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosas I.O., Ren P., Avila N.A., Chow C.K., Franks T.J., Travis W.D., McCoy J.P., Jr., May R.M., Wu H.-P., Nguyen D.M., Arcos-Burgos M., MacDonald S.D., Gochuico B.R. Early interstitial lung disease in familial pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2007;176:698–705. doi: 10.1164/rccm.200702-254OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Putman R.K., Hatabu H., Araki T., Gudmundsson G., Gao W., Nishino M., Okajima Y., Dupuis J., Latourelle J.C., Cho M.H., El-Chemaly S., Coxson H.O., Celli B.R., Fernandez I.E., Zazueta O.E., Ross J.C., Harmouche R., Estépar R.S.J., Diaz A.A., Sigurdsson S., Gudmundsson E.F., Eiríksdottír G., Aspelund T., Budoff M.J., Kinney G.L., Hokanson J.E., Williams M.C., Murchison J.T., MacNee W., Hoffmann U., O’Donnell C.J., Launer L.J., Harrris T.B., Gudnason V., Silverman E.K., O’Connor G.T., R.Washko G., Rosas I.O., Hunninghake G.M. Association between interstitial lung abnormalities and all-cause mortality. JAMA – J. Am. Med. Assoc. 2016;315:672–681. doi: 10.1001/jama.2016.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Araki T., Putman R.K., Hatabu H., Gao W., Dupuis J., Latourelle J.C., Nishino M., Zazueta O.E., Kurugol S., Ross J.C., Estépar R.S.J., Schwartz D.A., Rosas I.O., Washko G.R., O’Connor G.T., Hunninghake G.M. Development and progression of interstitial lung abnormalities in the Framingham Heart Study. Am. J. Respir. Crit. Care Med. 2016;194:1517–1522. doi: 10.1164/rccm.201512-2523OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Araki T., Dahlberg S.E., Hida T., Lydon C.A., Rabin M.S., Hatabu H., Johnson B.E., Nishino M. Interstitial lung abnormality in stage IV non-small cell lung cancer: a validation study for the association with poor clinical outcome. Eur. J. Radiol. Open. 2019;6:128–131. doi: 10.1016/j.ejro.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsushima K., Sone S., Yoshikawa S., Yokoyama T., Suzuki T., Kubo K. The radiological patterns of interstitial change at an early phase: over a 4-year follow-up. Respir. Med. 2010;104:1712–1721. doi: 10.1016/j.rmed.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 22.Podolanczuk A.J., Oelsner E.C., Barr R.G., Bernstein E.J., Hoffman E.A., Easthausen I.J., Stukovsky K.H., RoyChoudhury A., Michos E.D., Raghu G., Kawut S.M., Lederer D.J. High-attenuation areas on chest computed tomography and clinical respiratory outcomes in community-dwelling adults. Am. J. Respir. Crit. Care Med. 2017;196:1434–1442. doi: 10.1164/rccm.201703-0555OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris T.B., Launer L.J., Eiriksdottir G., Kjartansson O., Jonsson P.V., Sigurdsson G., Thorgeirsson G., Aspelund T., Garcia M.E., Cotch M.F., Hoffman H.J., Gudnason V. Age, gene/environment susceptibility-reykjavik study: multidisciplinary applied phenomics. Am. J. Epidemiol. 2007;165:1076–1087. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Putman R.K., Gudmundsson G., Axelsson G.T., Hida T., Honda O., Araki T., Yanagawa M., Nishino M., Miller E.R., Eiriksdottir G., Gudmundsson E.F., Tomiyama N., Honda H., Rosas I.O., Washko G.R., Cho M.H., Schwartz D.A., Gudnason V., Hatabu H., Hunninghake G.M. Imaging patterns are associated with interstitial lung abnormality progression and mortality. Am. J. Respir. Crit. Care Med. 2019;200:175–183. doi: 10.1164/rccm.201809-1652OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walsh S.L.F., Sverzellati N., Devaraj A., Wells A.U., Hansell D.M. Chronic hypersensitivity pneumonitis: high resolution computed tomography patterns and pulmonary function indices as prognostic determinants. Eur. Radiol. 2012;22:1672–1679. doi: 10.1007/s00330-012-2427-0. [DOI] [PubMed] [Google Scholar]
- 27.Jacob J., Aksman L., Mogulkoc N., Procter A.J., Gholipour B., Cross G., Barnett J., Brereton C.J., Jones M.G., van Moorsel C.H., van Es W., van Beek F., Veltkamp M., Desai S.R., Judge E., Burd T., Kokosi M., Savas R., Bayraktaroglu S., Altmann A., Wells A.U. Serial CT analysis in idiopathic pulmonary fibrosis: comparison of visual features that determine patient outcome. Thorax. 2020;75:648–654. doi: 10.1136/thoraxjnl-2019-213865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staats P., Kligerman S., Todd N., Tavora F., Xu L., Burke A. A comparative study of honeycombing on high resolution computed tomography with histologic lung remodeling in explants with usual interstitial pneumonia. Pathol. Res. Pract. 2015;211:55–61. doi: 10.1016/j.prp.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 29.Johkoh T., Sumikawa H., Fukuoka J., Tanaka T., Fujimoto K., Takahashi M., Tomiyama N., Kondo Y., Taniguchi H. Do you really know precise radiologic-pathologic correlation of usual interstitial pneumonia? Eur. J. Radiol. 2014;83:20–26. doi: 10.1016/j.ejrad.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 30.Raghu G., Remy-Jardin M., Myers J.L., Richeldi L., Ryerson C.J., Lederer D.J., Behr J., Cottin V., Danoff S.K., Morell F., Flaherty K.R., Wells A., Martinez F.J., Azuma A., Bice T.J., Bouros D., Brown K.K., Collard H.R., Duggal A., Galvin L., Inoue Y., Gisli Jenkins R., Johkoh T., Kazerooni E.A., Kitaichi M., Knight S.L., Mansour G., Nicholson A.G., Pipavath S.N.J., Buendía-Roldán I., Selman M., Travis W.D., Walsh S., Wilson K.C. Diagnosis of idiopathic pulmonary fibrosis an official ATS/ERS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med. 2018;198:e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 31.Miller E.R., Putman R.K., Vivero M., Hung Y., Araki T., Nishino M., Washko G.R., Rosas I.O., Hatabu H., Sholl L.M., Hunninghake G.M. Histopathology of interstitial lung abnormalities in the context of lung nodule resections. Am. J. Respir. Crit. Care Med. 2018;197:955–958. doi: 10.1164/rccm.201708-1679LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piciucchi S., Tomassetti S., Ravaglia C., Gurioli C., Gurioli C., Dubini A., Carloni A., Chilosi M., Colby T.V., Poletti V. From “traction bronchiectasis” to honeycombing in idiopathic pulmonary fibrosis: a spectrum of bronchiolar remodeling also in radiology? BMC Pulm. Med. 2016;16:87. doi: 10.1186/s12890-016-0245-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chilosi M., Poletti V., Murer B., Lestani M., Cancellieri A., Montagna L., Piccoli P., Cangi G., Semenzato G., Doglioni C. Abnormal re-epithelialization and lung remodeling in idiopathic pulmonary fibrosis: the role of deltaN-p63. Lab. Invest. 2002;82:1335–1345. doi: 10.1097/01.LAB.0000032380.82232.67. [DOI] [PubMed] [Google Scholar]
- 34.Walsh S.L.F., Wells A.U., Sverzellati N., Devaraj A., von der Thüsen J., Yousem S.A., Colby T.V., Nicholson A.G., Hansell D.M. Relationship between fibroblastic foci profusion and high resolution CT morphology in fibrotic lung disease. BMC Med. 2015;13:241. doi: 10.1186/s12916-015-0479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoyer N., Thomsen L.H., Wille M.M.W., Wilcke T., Dirksen A., Pedersen J.H., Saghir Z., Ashraf H., Shaker S.B. Increased respiratory morbidity in individuals with interstitial lung abnormalities. BMC Pulm. Med. 2020;20:67. doi: 10.1186/s12890-020-1107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.