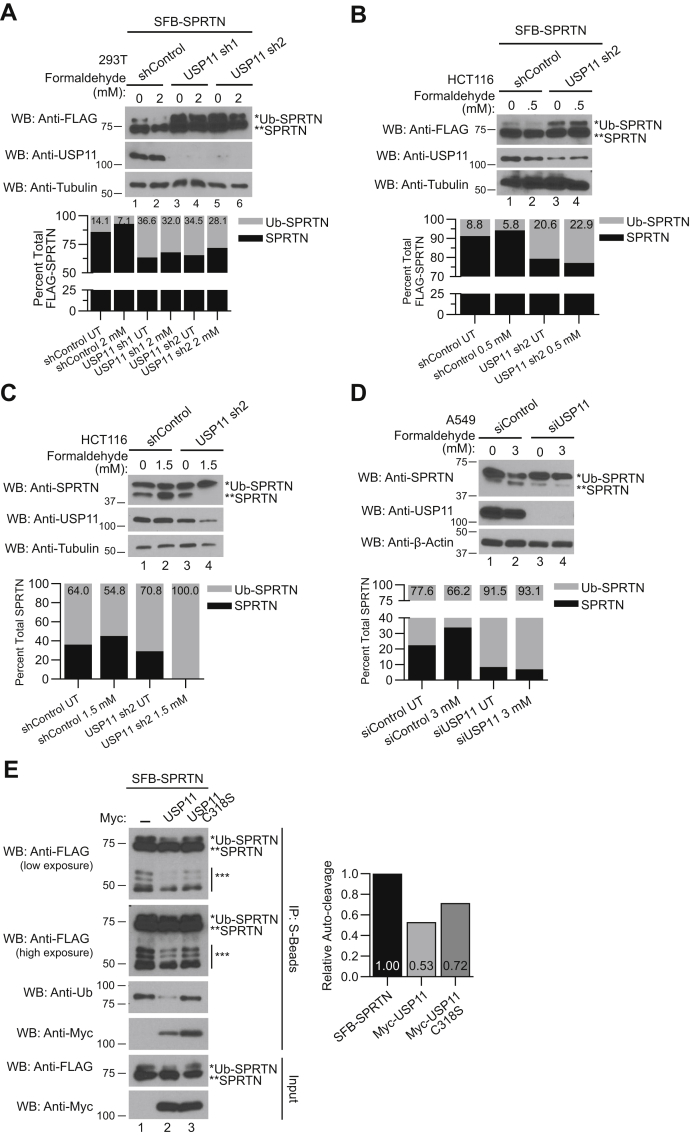
Figure 5.
USP11 deubiquitinates SPRTN upon DPC induction.A and B, HEK 293T (A) or HCT116 (B) cells stably expressing shRNA targeted to USP11 were transfected with SFB-SPRTN. Twenty-four hours later, cells were treated with the indicated concentrations of formaldehyde for 2 h. C, HCT116 cells stably expressing shRNA targeted to USP11 were treated with the indicated concentrations of formaldehyde for 1 h. The nuclear fraction was isolated and used for WB. D, A549 cells were transfected with siRNA targeted to USP11. Seventy-two hours later, cells were treated with the indicated concentrations of formaldehyde for 2 h. A–D, Top panel, cell lysates were immunoblotted with the indicated antibodies. Bottom panel, graph shows percent total FLAG-SPRTN or percent total SPRTN quantified from anti-FLAG or anti-SPRTN blots, respectively. The percent of unmodified and monoubiquitinated SPRTN was calculated. E, HEK 293T cells were transfected with SFB-SPRTN alone or in combination with Myc-USP11 FL or C318S mutant. Left panel, cell lysates were immunoprecipitated with S-protein agarose beads, and immunoblotting was performed using the indicated antibodies. Anti-Ub blot was probed with K63 ubiquitin antibody. Right panel, graph showing quantification of SFB-SPRTN auto-cleavage products. Anti-FLAG high exposure blot was used for quantification. WB, western blot. See also Figs. S4, S5, and S6.