Abstract
Latinas are less likely to participate in genetic counseling (GC) and genetic testing (GT) than non-Hispanic Whites. A multi-site, randomized pilot study tested a culturally targeted educational intervention to increase uptake of GC/GT among Latina breast cancer (BC) survivors (N=52). Participants were recruited in Tampa, FL and Ponce, PR and randomized to: (1) fact sheet about BC survivorship (control), or (2) a culturally targeted educational booklet about GC/GT (intervention). Participants in the intervention condition were also offered no-cost telephone GC followed by free GT with mail-based saliva sample collection. Participants self-reported hereditary breast and ovarian cancer (HBOC) knowledge and emotional distress at baseline and 1- and 3-month follow-ups. We used logistic regression to examine differences in GC/GT uptake by study arm (primary outcome) and repeated-measures ANOVA to examine the effects of study arm and time on HBOC knowledge and emotional distress (secondary outcomes). Compared to the control arm, intervention participants were more likely to complete GC (ORIntervention=13.92, 95% CI=3.06–63.25, p<0.01) and GT (ORIntervention=12.93, 95% CI=2.82–59.20, p<0.01). Study site did not predict uptake of GC (p=0.08) but Ponce participants were more likely to complete GT (ORPonce=4.53, 95% CI=1.04–19.72, p=0.04). ANOVAs demonstrated an increase in HBOC knowledge over time across both groups (F(2,88)=12.24, p<0.01, ηp2=0.22). We also found a significant interaction of study arm and time, such that intervention participants demonstrated a greater and sustained (to the 3-month follow-up) increase in knowledge than control participants (F(2,88)=3.66, p=0.03, ηp2=0.08). No other main or interaction effects were significant (all p’s>0.15). Study findings demonstrate the potential of our culturally targeted print intervention. Lessons learned from this multi-site pilot study for enhancing GC/GT in Latinas include the need to attend to both access to GC/GT and individual factors such as attitudes and knowledge.
Keywords: genetic counseling, genetic testing, educational intervention, Hispanic, Latina, breast cancer
Background
Breast cancer (BC) is the leading cause of cancer death among Latinas (American Cancer Society, 2020). Latinas are often diagnosed with BC at younger ages and with worse prognostic features (e.g., triple-negative disease) than non-Hispanic Whites (NHWs) (Boyle & McPadden, 2004; Lara‐Medina et al., 2011; Miranda et al., 2011). Genetic factors may contribute to these disparities (Fejerman et al., 2014; Lynce et al., 2016; Rey-Vargas et al., 2019; Weitzel et al., 2013). Multiple genes confer increased risk for breast and/or ovarian cancer (Lindor et al., 2008), with pathogenic variants in the BRCA1/2 genes being most prevalent and penetrant (Euhus & Robinson, 2013). The prevalence of pathogenic BRCA1/2 variants among Latinas is at least the same as NHW females of non-Ashkenazi Jewish ancestry (John et al., 2007; Weitzel et al., 2013). Prevalence rates are even higher in select clinical populations based on family history, clinical characteristics, cancer site, country of origin, and type of mutation (Dutil et al., 2015).
Although professional organizations provide guidelines to identify those at increased hereditary cancer risk (Daly et al., 2017; Khatcheressian et al., 2013), an estimated 300,000 United States (US) females carry a pathogenic BRCA1/2 variant and are unaware of their status (Drohan et al., 2012; Gross et al., 2018). Genetic counseling (GC) and genetic testing (GT) for BC survivors is critically important, as results may inform risk management for second primary cancers and targeted cascade testing for at-risk family members (National Comprehensive Cancer Network (NCCN), 2019). However, only ~15% of eligible BC survivors have undergone GT (Childers et al., 2017). Despite a strong desire for GC/GT, Spanish-speaking Latina BC survivors are less than half as likely as NHW survivors to report discussing GC/GT with a health provider (Cragun et al., 2017; Jagsi et al., 2015). Providers may not refer high-risk Latinas for GC due to concerns about access, language and cultural barriers (Hurtado-de-Mendoza et al., 2018). Given these barriers, the percentage of Latinas unaware of their hereditary cancer risk is likely quite high (Childers et al., 2017; Jagsi et al., 2015; Levy et al., 2011).
Nationally, ~60% of Latinos age ≥18, 68% of foreign-born Latinos, and 72% of people in Puerto Rico (PR) rated their English ability below “very well” (Krogstad & Gonzalez-Barrera, 2015). Even Latinos who are highly proficient in English may prefer receiving complex health information in Spanish (Kaplan et al., 2016; Kinney et al., 2010; McIntyre et al., 2017; Quinn et al., 2013). Access to bilingual GC for Spanish-preferring patients remains a barrier for Latinas in mainland US and PR (Cruz-Correa et al., 2017). Nationwide, very few (~6%) genetic counselors speak fluent Spanish (Augusto et al., 2019). Growing interest exists in developing alternative GC delivery models to increase access to underserved groups (Buchanan et al., 2016; Stoll et al., 2018), including telephone delivery of GC (Kinney et al., 2016; Schwartz et al., 2014). However, few efforts have focused on Spanish-preferring patients.
Prior work by our group and others has demonstrated no differences in knowledge of or interest in GC/GT between Latinas in Ponce, PR and Tampa, FL (Vadaparampil et al., 2011). However, there are differences in the availability and delivery of genetics services in the mainland US and PR. In the mainland US, there are few Spanish-speaking genetic counselors. While there is a significantly higher percentage of Spanish-speaking genetic counselors in states with larger Latino populations, the absolute number is low and unlikely to meet the needs of patients (Augusto et al., 2019). Currently, clinical cancer genetic services in Latin America are scarce; in PR, a small number of clinics – mainly in the largest cities – offer clinical cancer GC (Cruz-Correa et al., 2017). Despite equivalence in knowledge of and desire for GC/GT, these structural factors may necessitate unique intervention approaches to promote GC/GT uptake. Additional multisite studies are needed to better understand the unique factors that affect GC/GT among Latinas in the mainland US versus PR.
To address this gap, we used a culturally targeted, English-language, print intervention about GC/GT and refined it for high-risk, Spanish-preferring BC survivors (Quinn et al., 2011). In order to determine whether this novel intervention resulted in a clinically significant benefit for Latina BC survivors, we designed and executed a Phase IIb pilot study (per the Obesity-Related Behavioral Intervention Trials (ORBIT) model for behavioral treatment development; Czajkowski et al., 2015). We recruited BC survivors in Florida and PR and randomized them to our culturally targeted intervention or a control condition. We assessed whether our culturally targeted intervention increased GC/GT uptake. Secondary outcomes included hereditary breast and ovarian cancer (HBOC) knowledge and emotional distress. We also share “lessons learned” about community-level factors that varied between the US and PR and impacted study procedures.
Methods
Study Design
This was a multicenter, randomized (1:1), parallel-group pilot study (see supplemental materials for CONSORT checklist). The target sample size was 25 participants per study arm, based on sample size recommendations for pilot studies (Cocks & Torgerson, 2013; Hertzog, 2008; Schoenfeld, 1980).
Participants and Procedures
The Moffitt Cancer Center (MCC; IRB #18601) and Ponce Health Sciences University-Ponce Research Institute (PHSU; IRB #160607-EC) Institutional Review Boards approved all study procedures. Eligible participants were female BC patients who were: 1) self-identified Hispanic/Latina; 2) age ≥25; 3) Spanish-speaking; 4) able to provide informed consent; 5) GC/GT-naïve; and 6) eligible for genetics referral based on 2017 NCCN Guidelines (Daly et al., 2017). Specifically, patients met criteria for genetics referral if they had: 1) a known pathogenic variant in a cancer susceptibility gene within the family; 2) BC diagnosis before age 50; 3) triple-negative BC diagnosis before age 60; 4) two primary BC diagnoses; 5) ≥1 close blood (1st, 2nd, or 3rd degree) relative with BC before age 50; 6) ≥2 close blood relatives from the same side of the family with BC or pancreatic cancer, 7) a personal or family history of ovarian cancer, or 8) a personal history of pancreatic cancer. In this manuscript, we refer to patients meeting one or more of these criteria as “high-risk”. Women age <25 were not eligible for the present study as evidence-based HBOC risk management options that would typically be considered post-GC/GT (e.g., radiographic screening, risk-reducing mastectomy, risk-reducing salpingo-oophorectomy) are generally not recommended until individuals are 25 years old (Bradbury et al., 2012; Robson & Offit, 2007).
At MCC, the provision of GC/GT services required that participants have a healthcare provider (HCP) to receive GT results. Because our sample was community-based (i.e., not MCC patients), the MCC providers ordering GT felt it was important to provide GT results to a HCP of the participant’s choosing, who would be familiar with their care. Participants had the option to list any HCP, irrespective of specialty. Given the potential for GT results to inform medical management for study participants, this was an additional criterion for eligibility at MCC. PHSU did not require participants to have a HCP to receive GT results.
We recruited participants in the Tampa Bay area (West Central Florida) and Ponce Region (Southern PR) through institutional and community based clinics and registries, community sites (e.g., community oncology clinics, cancer support groups) and outreach activities (e.g., cancer education events, health fairs), Spanish language media channels, and social media sites using our robust community outreach infrastructure (Gwede et al., 2012). Recruitment took place from January to June 2017. Individuals interested in participation called a toll-free telephone number to learn more about the study. A research assistant screened callers for eligibility and mailed eligible individuals two copies of the informed consent and a business reply mail envelope. During in-person recruitment (e.g., at community events, clinic), a research assistant immediately screened and consented potential participants face-to-face. We continued accrual until we reached the target sample size (n = 50 [25 per arm]).
Study Design and Intervention
Following informed consent, participants completed a baseline (T1) survey in Spanish either in person, via mail, or by telephone (depending on participant preference). Upon completion of the baseline survey, the site PI randomized participants to intervention or control groups using block randomization in 1:1 fashion. The study team generated the randomization list using “proc plan” in SAS (Cary, NC) and used a random numbers table to assign participants to study arms. Randomization was stratified by site to ensure equal representation of study arms at each study site. To ensure allocation concealment, separate study team members at each site enrolled participants and assigned participants to study arms. While investigators were aware of the allocated arm, participants and genetic counselors were kept blinded to the allocation.
Intervention group participants received a culturally and linguistically targeted educational booklet adapted from a brief English brochure about GC/GT (Quinn et al., 2011). The research team reviewed the existing brochure to verify content, cultural relevance, visual appeal, and style appropriate for low literacy individuals. After adding additional content regarding GC (definition, process, and benefits) and GT (process, timeframe, and financial aid resources), the booklet was translated from English to Spanish using a certified translator, and revised by a bilingual genetic counselor and research coordinators to ensure language was appropriate for the Latina communities in Tampa and Ponce. A professional design company formatted the booklet to highlight: a) the importance of GC/GT for Latina BC survivors and their families; b) HBOC risk factors; and c) availability of telephone GC/GT. Finally, 10 high-risk BC survivors (5 in PR and 5 in Tampa; reviewers were not eligible for subsequent study participation) reviewed the booklet for attractiveness, comprehensibility, cultural acceptability, and persuasiveness using principles of learner verification (Chavarria et al., in press). They regarded the booklet favorably with minimal suggestions for improvement. The finalized booklet covered the following topics: 1) “What is hereditary breast cancer?” (including an example of a pedigree that is significant for HBOC); 2) “How would I find out if I carry a gene mutation?”; 3) “What is genetic counseling?”; 4) “How can genetic counseling help me and my family?”; 5) “How is genetic testing done?”; and 6) “What are some signs hereditary cancer may run in my family?” (including a family history questionnaire for self-assessment of risk factors). We mailed the finalized booklet to participants in the intervention group, along with a postcard to request telephone-based GC/GT at no cost, coordinated by the study team.
Through the study, participants in the intervention group had access to no-cost GC and GT for HBOC. A partner organization provided both GC and GT. Pre- and post-test telephone GC was conducted either through a bilingual genetic counselor or with the assistance of a medical interpreter based on participant’s preference and counselor availability. After pre-test GC, participants had the option to obtain GT; those interested were mailed an Oragene DNA Collection Kit (saliva samples). A CLIA-approved testing laboratory provided GT. The GT panel included the following genes: ATM, BRCA1, BRCA2, BRIP1, CDH1, CHEK2, EPCAM (deletion/duplication testing only), MLH1, MSH2, MSH6, NBN, NF1, PALB2, PMS2, PTEN, RAD51C, RAD51D, STK11, and TP53. Genetic counselors disclosed participants’ GT results in post-test GC; results were also uploaded to the patient’s portal on the commercial testing laboratory website. Finally, the study team sent a letter to the participants who completed GT encouraging them to share their results with their providers.
Identical to usual care at both sites, participants in the control group did not have direct access to no-cost GC and GT for HBOC. Rather, the control group received a one-page factsheet in Spanish with nine questions to ask a health professional regarding BC survivorship (e.g., “How often should I be coming in for my follow up appointments?”, “Should I continue to have an annual mammogram?, etc.) (Ashing et al., 2013). One of these questions referred to GC (“Does my personal and/or family history indicate the need for genetic counseling?”), but no further information about GC was provided. The fact sheet was accompanied by a postcard to request a survivorship care plan. Availability of supplementary project funds allowed for the Ponce team to offer no-cost GC/GT to participants assigned to the control condition, after the conclusion of the study follow-up period. If participants from Ponce completed GC/GT outside of the study time period, we did not include those results in the primary analyses. However, uptake of GC/GT in the Ponce control group was assessed as part of exploratory analyses.
Finally, participants completed follow-up assessments in Spanish via mail 1-month post-randomization (T2) and 3-months post-randomization (T3).
Measures
We collected data via self-report (T1 in person, via mail, or by telephone; T2 and T3 via mail only) and laboratory reports. Participants completed a release of information allowing the research team to review medical records related to GC and GT. A certified Spanish language translator reviewed and revised all self-report measures as needed.
Sociodemographic, disease, and treatment characteristics (T1).
Participants self-reported age, race, birthplace, relationship status, education, employment status, income, insurance status, date and age of cancer diagnosis, cancer stage, and treatment(s) received (surgery, chemotherapy, radiation therapy, hormonal therapy).
Primary Outcomes: GC/GT Uptake.
The primary outcomes of interest were GC/GT uptake. A research assistant verified GC status by reviewing data from the partner organization that provided telephone-based GC, and abstracted GT status from reports from the commercial testing laboratory. At Moffitt, GC/GT uptake occurred entirely within the study period. At Ponce, GC/GT uptake was categorized as occurring either during the study period (up to T3) or after the study period (after T3). GC/GT uptake during the study period was the primary outcome of interest; uptake following the study period was used for exploratory analyses.
Secondary Outcomes: Knowledge and Emotional Distress (T1, T2, T3).
Secondary outcomes included: (1) HBOC Knowledge. We assessed HBOC knowledge using a 15-item version of the National Center for Human Genome Research Knowledge scale modified to include items specific to BC survivors (Scherr et al., 2016). Correct responses are summed to create a total HBOC knowledge score (range: 0–15). (2) Emotional Distress. The Hospital Anxiety and Depression Scale (HADS)(Zigmond & Snaith, 1983) has anxiety and depression subscales with 7 items each. Participants rate items on a 4-point Likert scale (0 to 3), and items are summed for subscale scores ranging from 0–21. Higher scores indicate more symptoms, and the recommended clinical cutoff is ≥8 (Zigmond & Snaith, 1983).
Data Analysis
First, chi-square analyses (for categorical variables) or two-sample t-tests (for continuous variables) examined whether sociodemographic and medical history variables differed by study arm or study site. Second, multiple logistic regression models examined differences in GC/GT uptake by study arm. We entered study site (i.e., Tampa v. Ponce) as a covariate in the logistic regression models in order to adjust for and to estimate the site effect. These analyses were intention-to-treat and involved all patients who were randomly assigned. Third, repeated measures ANOVA with list-wise deletion examined the effects of study arm, time, and the interaction of study arm and time on HBOC knowledge, depression, and anxiety. Finally, exploratory analyses using the exact test by binomial distribution tested whether uptake of GC/GT after the study period in the Ponce control group significantly differed from uptake of GC/GT in the intervention arm during the study period. The null hypothesis was that there is no difference between uptake of GC/GT in these two groups during the specified time period, which can be specified as H0: θ = X against Ha: θ ≠ X. Here, θ represents the proportion of GC/GT uptake after the study period in the Ponce control group, and X represents the uptake of GC/GT in the intervention arm during the study period. All p-values are two-sided and significance was specified as p<0.05. All analyses were conducted in IBM SPSS version 25 (IBM Corp, 2017).
Results
Descriptive and Preliminary Analyses
Across sites, we assessed 82 BC patients for eligibility (Figure 1); 66 (80%) met inclusion criteria, and 52 (79%) consented, completed the T1 survey, and were randomized (n=26 per study arm). Of the randomized participants, 28 (54%) were from Tampa and 24 (46%) were from Ponce.
Figure 1:
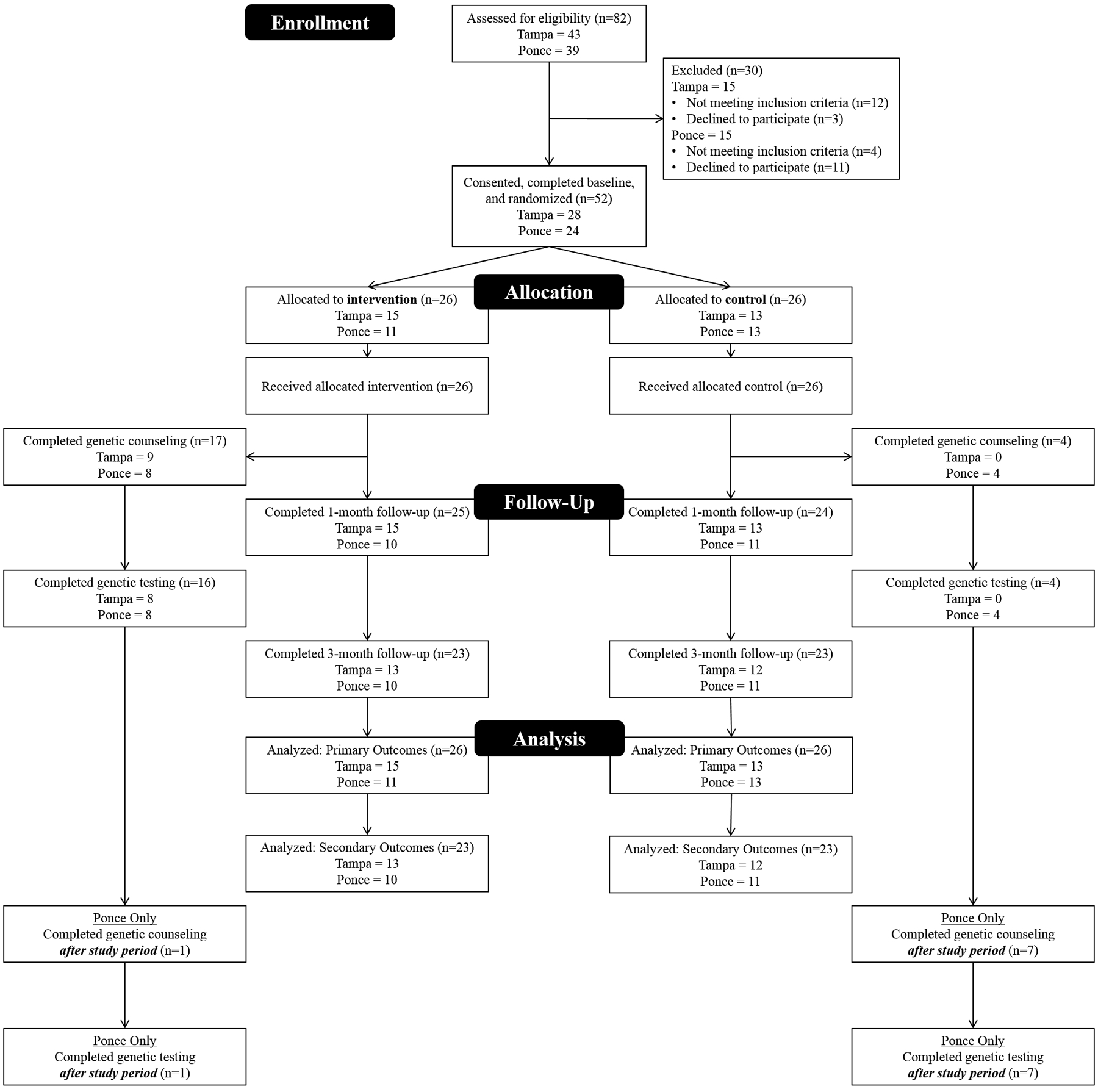
CONSORT Diagram.
Participants’ mean age was 54 years (SD=9 years) and 64% of participants were partnered (Table 1). Regarding birthplace, 56% were born in PR, 21% in Colombia, and 17% in Cuba. The majority had at least a high school education (87%) and medical insurance (89%). Participants were an average of 7 years post-BC diagnosis (SD=5 years). Most reported a local (Stage 0-II) BC diagnosis (52%) and receiving surgery (94%), chemotherapy (79%), and radiation (58%). About one-third (33%) was currently taking hormonal therapy.
Table 1:
Sample characteristics by study site and intervention group (N=52).
| Tampa, FL | Ponce, PR | Tampa v. Ponce: p | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Overall (n=28) | Intervention (n=15) | Control (n=13) | Int v. Control: p | Overall (n=24) | Intervention (n=11) | Control (n=13) | Int v. Control: p | |
| Age (M, SD) | 54.9 (7.0) | 53.4 (7.0) | 56.6 (6.9) | 0.23 | 53.5 (10.7) | 52.8 (12.2) | 54.0 (9.8) | 0.80 | 0.57 |
| Partnered (N, %) | 20 (71) | 12 (80) | 8 (62) | 0.28 | 13 (54) | 5 (45) | 8 (62) | 0.43 | 0.20 |
| Education (N, %) | 0.63 | 0.86 | 0.53 | ||||||
| <High School/GED | 3 (11) | 2 (13) | 1 (8) | 4 (17) | 2 (18) | 2 (15) | |||
| ≥High School/GED | 25 (89) | 13 (87) | 12 (92) | 20 (83) | 9 (82) | 11 (85) | |||
| Household Income (N, %) | 0.78 | 1.00 | 0.17 | ||||||
| <$35,000/year | 18 (64) | 10 (67) | 8 (62) | 18 (75) | 9 (82) | 9 (69) | |||
| ≥$35,000/year | 10 (36) | 5 (33) | 5 (38) | 4 (17) | 2 (18) | 2 (15) | |||
| Insured (N, %) | 23 (82) | 14 (93) | 9 (69) | 0.10 | 23 (96) | 11 (100) | 12 (92) | 0.35 | 0.12 |
| Birthplace (N, %) | 1.00 | 0.27 | <0.01* | ||||||
| Colombia | 11 (39) | 6 (40) | 5 (38) | 0 (0) | 0 (0) | 0 (0) | |||
| Cuba | 9 (32) | 5 (33) | 4 (31) | 0 (0) | 0 (0) | 0 (0) | |||
| Puerto Rico | 6 (21) | 3 (20) | 3 (23) | 23 (96) | 10 (91) | 13 (100) | |||
| Mainland US | 0 (0) | 0 (0) | 0 (0) | 1 (4) | 1 (9) | 0 (0) | |||
| Other | 2 (7) | 1 (7) | 1 (8) | 0 (0) | 0 (0) | 0 (0) | |||
| Years Since Diagnosis (M, SD) | 7.8 (4.7) | 8.8 (4.7) | 6.6 (4.5) | 0.23 | 5.4 (4.1) | 3.6 (2.2) | 6.9 (4.8) | 0.05* | 0.06 |
| Cancer Stage (N, %) | 0.57 | 0.10 | 0.52 | ||||||
| Local (0-II) | 14 (50) | 9 (60) | 5 (38) | 13 (54) | 5 (45) | 8 (62) | |||
| Regional (III) | 6 (21) | 3 (20) | 3 (23) | 3 (13) | 0 (0) | 3 (23) | |||
| Distant (IV) | 4 (14) | 2 (13) | 2 (15) | 1 (4) | 1 (9) | 0 (0) | |||
| Don’t Know | 4 (14) | 1 (7) | 3 (23) | 5 (21) | 4 (36) | 1 (8) | |||
| Missing | 0 (0) | 0 (0) | 0 (0) | 2 (8) | 1 (9) | 1 (8) | |||
| Treatment (N, %) | |||||||||
| Surgery | 26 (93) | 14 (93) | 12 (92) | 0.29 | 23 (96) | 10 (91) | 13 (100) | 0.27 | 0.93 |
| Chemotherapy | 22 (79) | 15 (100) | 7 (54) | <0.01* | 19 (79) | 9 (82) | 10 (77) | 0.77 | 0.96 |
| Radiation Therapy | 15 (54) | 9 (60) | 6 (46) | 0.46 | 15 (53) | 7 (64) | 8 (62) | 0.92 | 0.52 |
| Hormonal Therapy | 9 (32) | 10 (67) | 9 (69) | 0.89 | 8 (33) | 5 (45) | 3 (23) | 0.22 | 0.54 |
p<0.05
In Tampa, the only significant difference between intervention and control groups was receipt of chemotherapy (p<0.01), such that participants in the intervention group were more likely to have received chemotherapy than participants in the control group (100% v. 54%). In Ponce, the only significant difference between intervention and control groups was time since BC diagnosis (p=0.05), such that participants in the intervention group were diagnosed more recently than participants in the control group (3.6 years v. 6.9 years). No other sociodemographic, disease, or treatment characteristics differed between the intervention and control groups in Tampa or Ponce (all p’s>0.10). Comparing across study sites, Ponce participants were more likely than Tampa participants to report PR as their birthplace (p<0.01). There were no other significant differences between Tampa and Ponce participants in sociodemographic or clinical characteristics (all p’s>0.06).
Primary Outcomes
Across sites, 17 of 26 participants allocated to the intervention group (65%) completed GC. Of those, 16 (94%) subsequently completed GT. Nine participants (56%) received negative GT results. GT identified variants for 7 participants (44%) in the intervention group; 6 were variants of uncertain significance and 1 was pathogenic (Table 2). In the control group, 4 of 26 participants (15%) completed GC. Of those, 4 (100%) subsequently completed GT. Two participants (50%) received negative GT results. GT identified variants for 2 participants (50%) in the control group; both variants were of uncertain significance (Table 2).
Table 2:
Variants identified in participants completing GT (n=20).
| Variant Classification | |||||||
|---|---|---|---|---|---|---|---|
| Site | Study Arm | Study ID | Birthplace | Gene | Variant | Commercial Lab Classification | Current ClinVar† Classification |
| Tampa | Intervention | 03 | Puerto Rico | CDH1 | c.1568A>G (p.Tyr523Cys) | VUS | VUS |
| 09 | Dominican Republic | N/A | Negative | N/A | N/A | ||
| 11 | Cuba | MSH2 | c.138C>G (p.His46Gln) | VUS | Likely Benign | ||
| 16 | Colombia | MSH6 | c.3757G>A (p.Val1253Ile) | VUS | VUS | ||
| 23 | Colombia | BRIP1 | c.1825A>G (p.Thr609Ala) | VUS | VUS | ||
| 25 | Cuba | CHEK2 | c.715G>T (p.Glu239*) | Pathogenic | Pathogenic | ||
| 27 | Puerto Rico | N/A | Negative | N/A | N/A | ||
| 28 | Colombia | N/A | Negative | N/A | N/A | ||
| Ponce | Intervention | 62 | Puerto Rico | N/A | Negative | N/A | N/A |
| 63 | Puerto Rico | N/A | Negative | N/A | N/A | ||
| 66 | Puerto Rico | N/A | Negative | N/A | N/A | ||
| 67 | Puerto Rico | ATM | c.7740A>C (p.Arg2580Ser) | VUS | *Conflicting interpretations | ||
| 69 | Mainland US | CHEK2 | c.663C>G (p.Ile221Met) | VUS | VUS | ||
| 72 | Puerto Rico | N/A | Negative | N/A | N/A | ||
| 73 | Puerto Rico | N/A | Negative | N/A | N/A | ||
| 77 | Puerto Rico | N/A | Negative | N/A | N/A | ||
| Control | 76 | Puerto Rico | NBN | c.1999T>C (p.Ser667Pro) | VUS | *Conflicting interpretations | |
| 78 | Puerto Rico | MSH6 | c.3071G>A (p.Arg1024Gln) | VUS | VUS | ||
| 81 | Puerto Rico | N/A | Negative | N/A | N/A | ||
| 83 | Puerto Rico | N/A | Negative | N/A | N/A | ||
VUS = Variant of Uncertain Significance
N/A = not applicable
National Center for Biotechnology Information. ClinVar. Retrieved from https://www.ncbi.nlm.nih.gov/clinvar/. Accessed April 8, 2020.
Conflicting interpretations of pathogenicity on ClinVar, “likely benign” vs. “VUS”
Participants in the intervention arm were significantly more likely than participants in the control group to complete GC (ORIntervention=13.92, 95% CI=3.06–63.25, p<0.01) and GT (ORIntervention=12.93, 95% CI=2.82–59.20, p<0.01) (Table 3). Study site did not predict uptake of GC (p=0.08) but did predict uptake of GT, such that Ponce participants were more likely to complete GT (ORPonce=4.53, 95% CI=1.04–19.72, p=0.04).
Table 3:
Results of multiple logistic regressions examining predictors of GC/GT (N=52).
| Predictors | GC | GT |
|---|---|---|
| Study Arm | ||
| Control | (ref) | (ref) |
| Intervention | 13.92* [3.06, 63.25] | 12.93* [2.82, 59.20] |
| Study Site | ||
| Tampa, FL | (ref) | (ref) |
| Ponce, PR | 3.70 [0.85, 16.02] | 4.54* [1.04, 19.72] |
p<0.05
Secondary Outcomes
Repeated measures ANOVA demonstrated a significant main effect of time on HBOC knowledge (Figure 2), such that HBOC knowledge increased over time for participants in both study arms (F(2,88) = 12.24, p<0.01, ηp2=0.22). We found a significant interaction effect of study arm and time, such that participants in the intervention arm demonstrated greater and sustained increases in knowledge than participants in the control arm (F(2,88) = 3.66, p=0.03, ηp2=0.08). There was no main effect of study arm on HBOC knowledge (p=0.64). In addition, there were no significant main or interaction effects of study arm and time for depression (all p’s>0.35) or anxiety (all p’s>0.15) (Figure 2).
Figure 2:
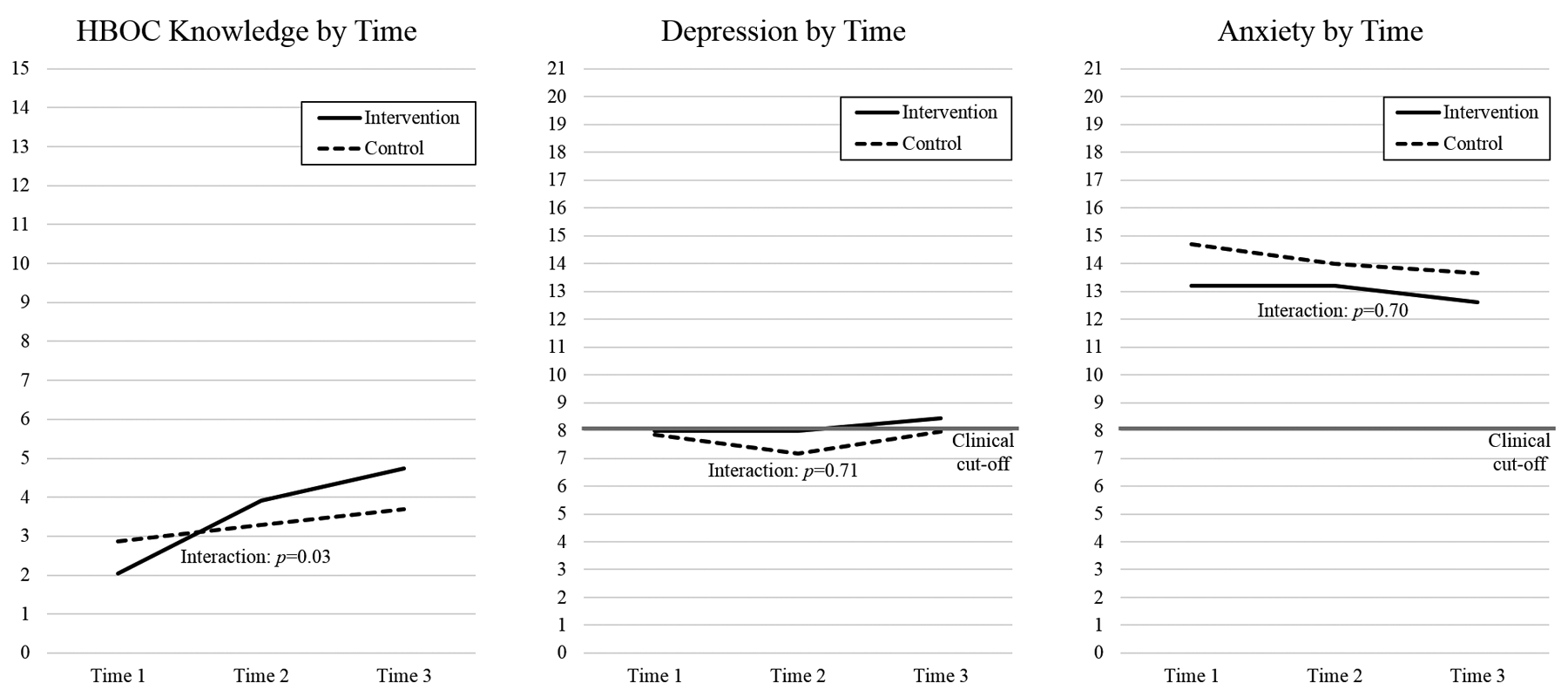
Secondary outcomes (HBOC knowledge, depression, and anxiety) by time and study arm (n=46). HBOC knowledge demonstrated a significant main effect of time and a significant interaction effect of study arm and time. There were no significant main or interaction effects for depression or anxiety.
Exploratory Outcomes
After the completion of the study period, 7 participants from the Ponce control group completed both GC and GT. They represented 54% of those randomized to the control group at Ponce. When accounting for participants in the control arm who had completed GC/GT during the study period (n=4), 78% of the remaining untested control group participants from Ponce completed GC/GT after the end of the study.
Based on the observed uptake of GC and GT in the intervention arm during the study period, the exact test by binomial distribution tested whether uptake of GC after the study period in the Ponce control group (78%) significantly differed from 65% (i.e., H0: θ = 0.65 against Ha: θ ≠ 0.65). Rates of GC uptake did not significantly differ between these two groups during the specified time periods (p=0.34). Regarding uptake of GT, uptake of GC after the study period in the Ponce control group (78%) was compared to the observed rate of 62% in the intervention group (i.e., H0: θ = 0.62 against Ha: θ ≠ 0.62). Similar to the GC results, rates of GT uptake did not significantly differ between these two groups during the specified time periods (p=0.27).
Discussion
Despite the significant benefit conferred by GC/GT for high-risk BC survivors, uptake remains low (Childers et al., 2017). Rates of GC/GT are even lower for Spanish-speaking Latina BC survivors (Cragun et al., 2017; Jagsi et al., 2015). Possible explanations for this disparity include low provider referral and patient awareness, limited access, and language and cultural barriers to genetic services (Mai et al., 2014; Quinn et al., 2011). We examined whether a patient-directed, culturally targeted print intervention for high-risk Spanish-preferring BC survivors could affect uptake of GC/GT. The data presented demonstrate the potential of our culturally targeted print intervention and – perhaps more importantly – highlight the important role of access to GC/GT in uptake of these services.
We found preliminary evidence for our culturally targeted educational intervention in increasing GC/GT uptake among high-risk Spanish-preferring BC survivors in the context of no cost GC/GT. Participants in the intervention group were significantly more likely than participants in the control group to complete both GC and GT. However, these results must be interpreted with caution given the significant differences in GC/GT availability between the study arms. During the study period, free GC/GT through the study was only available to participants in the intervention arm. Thus, the increased rates of GC/GT in the intervention arm may be due to the convenient, low-burden provision of genetics services, rather than the culturally targeted educational intervention. The important role of access to GC/GT is also supported by our exploratory analyses, examining uptake of GC and GT in the Ponce control arm (who were offered no-cost GC/GT after the conclusion of the study follow-up period). Of the 9 participants in the Ponce control group who did not complete GC/GT during the study period, 7 (78%) completed GC/GT after the end of the study. This was not significantly different from uptake in the intervention arm during the study period. This suggests that convenient, low-cost access to GC/GT is critical for uptake of these services. By providing GC/GT free of charge and facilitating the logistics of scheduling and follow-up, participants demonstrated uptake of services that is much higher than that observed in some clinical contexts (Childers et al., 2017; Drohan et al., 2012; Gross et al., 2018). Our findings align with the limited studies that report on uptake of GT in Latinas in settings with facilitated access to testing (Komenaka et al., 2016; Olaya et al., 2009; Rana et al., 2018; Ricker et al., 2006; Woodson et al., 2015). This has important implications for clinical practice; interventions addressing systems-level barriers may be necessary to increase uptake of GC/GT in this population.
Regarding our secondary outcomes, there was a significant main effect of time on HBOC knowledge, such that participants in both the intervention and control arms demonstrated increased knowledge over time. The increase in HBOC knowledge in the intervention arm is likely due to the information-rich educational brochure that was provided to patients. While the factsheet provided to the control arm included minimal information about HBOC, it did include questions to ask a health professional regarding BC survivorship. These types of structured question lists (or “Question Prompt Lists [QPLs]”) are associated with increased question asking during medical appointments and increased recall of information following appointments (Brandes et al., 2015). Although not assessed in the present study, it is possible that our control condition facilitated patient-provider conversations regarding HBOC, subsequently affecting HBOC knowledge. In addition to the main effect of time, we also observed a significant interaction effect on knowledge about HBOC. As HBOC knowledge is a known predictor of GT uptake (Hurtado-de-Mendoza et al., 2017; Scherr et al., 2016), future studies might investigate HBOC knowledge as a mediator of this intervention’s effect on GC/GT uptake.
There were no significant main or interaction effects on psychosocial distress; this may be the result of our small sample size, little variability in distress levels, or the timing of our assessments. Notably, this sample demonstrated clinically significant levels of anxiety at all time points. This is consistent with prior literature demonstrating elevated levels of distress and anxiety among Latina BC survivors (Crane et al., 2019; Rush et al., 2016; Yanez et al., 2016). A recent systematic review reported that 20 studies have demonstrated an effect of GC on anxiety (Nelson et al., 2019). However, this effect depended on the GT results; anxiety was higher after GT for those with positive results and lower for those with negative results. In the present study, our sample size limited our ability to examine differences in anxiety and depression by GT result. The lack of change in anxiety over time may be attributable to our decision to collapse across groups for these analyses.
Finally, this study provides some “lessons learned” regarding study design and implementation, particularly across multiple sites with different restrictions and demands. Participants recruited in PR were more likely to complete GT when compared to participants recruited in Tampa. Notably, of participants recruited in Tampa, there was no difference between PR-born women and women from other Latino groups in GT completion (PR=33%, all others=27%, χ2=0.09, p=0.77). In combination with our prior work demonstrating no differences in knowledge of or interest in GC/GT between Latinas in Ponce, PR and Tampa, FL (Vadaparampil et al., 2011), these results suggest that the difference in GT completion is most likely attributable to setting and availability of services, rather than cultural characteristics, interest, or knowledge. This may be for a variety of reasons. First, institutional policies in Tampa required that participants have a healthcare provider who was able to receive copies of GT results. This excluded a significant number of Tampa-based individuals from study participation, and may have resulted in a sample that is not representative of the larger population of at-risk Latina BC survivors. Second, PR participants may have been more motivated to engage in study GC/GT given the limited availability of clinical cancer genetic services in PR. Thus, considering the larger social, demographic, and cultural context in which research is being conducted is critical for study design. Interventions in varying healthcare contexts – particularly interventions targeting genetics services – may require adaption to increase feasibility and acceptability for a target population and institution.
The multi-site nature of this study is a strength. This design enables us to make inferences about the role of institutional and system-level policies on individual outcomes. In addition, this study had a relatively high retention rate, with 90% of baseline participants completing the 3-month follow-up assessment. Finally, a partner organization provided GC/GT for participants in the intervention group, thereby standardizing information participants received regarding their personal risk, HBOC, and risk management. Thus, we have greater confidence that the knowledge and psychosocial outcomes observed were not related to differences in information provided during the process of GC/GT, as might be the case in a naturalistic study of changes after GC/GT provided outside of the research context.
However, study results should be interpreted in the context of some limitations. First, this study was a Phase IIb pilot study (Czajkowski et al., 2015), and our sample size (N=52) was based on sample size recommendations for pilot studies (Cocks & Torgerson, 2013; Hertzog, 2008; Schoenfeld, 1980). While this design enables us to assess whether our intervention produces a clinically significant signal (versus the control condition), a large-scale, Phase III randomized controlled trial is needed to demonstrate the efficacy of this intervention. Second, generalizability of study findings are limited due to the sample characteristics (all Latina BC survivors). Almost all participants in this study identified as Colombian, Cuban, and Puerto Rican; this cohort may not represent the differing cultural and historical perspectives of other Latino groups. Third, our convenience sample may be subject to selection bias; participants who choose to enroll in a study of GC/GT may be more positive toward GC/GT than the average population. In addition, this convenience sample was achieved via several different recruitment approaches. We did not systematically collect recruitment modality for each participants, and thus are unable to examine differences in GC/GT uptake by recruitment source (e.g., in-person versus via telephone). Fourth, GC was phone-based, which has been associated with lower uptake of GT (Kinney et al., 2016), particularly among racial/ethnic minority groups (Butrick et al., 2015). Finally, the follow-up time point selected (3 months) was short and may have limited our understanding of survivors’ final GC/GT decisions. Future studies with longer follow-up are needed to understand the impact of our culturally tailored psychoeducational intervention on BC risk management behaviors among Latina BC survivors.
Conclusions
Results from this study have clinical, practical, and scientific implications. A culturally-tailored psychoeducational intervention may increase uptake of GC/GT for Latina BC survivors; however, future dismantling studies are necessary to understand the relative impact of psychoeducation versus systems-level interventions (e.g., free GC/GT). A larger randomized controlled trial, examining these issues and demonstrating intervention efficacy, is being planned. Additionally, this study demonstrates the importance of considering potential implementation barriers prior to the conduct of randomized behavioral clinical trials; institution and system-level policies may have a far greater impact on behavioral outcomes than behavioral interventions alone. Thus, it behooves behavioral researchers to consider the context in which they are conducting their research and “design for dissemination” (Klesges et al., 2005).
Supplementary Material
Acknowledgements
Thank you to Roxana Maldonado for her assistance with this project. This work was supported in part by the Biostatistics Core Facility at the H. Lee Moffitt Cancer Center & Research Institute, an NCI-designated Comprehensive Cancer Center (P30CA076292; PI: Cleveland) and by grants from the National Cancer Institute (U54CA163071, PIs: Matta & Dutil; U54CA163068, PIs: Wright & Monteiro; T32CA090314, PIs: Brandon & Vadaparampil).
Footnotes
Conflict of Interest
The authors have no conflicts of interest to report.
Human Studies and Informed Consent
All procedures were approved by the Moffitt Cancer Center (IRB #18601) and Ponce Health Sciences University-Ponce Research Institute (IRB #160607-EC) Institutional Review Boards. This study confirms to the standards outlined in the Declaration of Helsinki and US Federal Policy for the Protection of Human Subjects. All persons gave their informed consent prior to inclusion in the study.
Data Availability: Anonymized data that support the findings of this study are available from the corresponding author, STV, upon reasonable request
References
- American Cancer Society. (2020). Cancer Facts & Figures. American Cancer Society. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf [Google Scholar]
- Ashing KT, Rosales M, Fulcher G, Serrano M, Weitzel JN, Paz IB, & Lai LL (2013). Developing a culturally and linguistically responsive survivorship care plan for breast cancer. J Clin Oncol, 31_suppl, 146–146. [Google Scholar]
- Augusto B, Kasting ML, Couch FJ, Lindor NM, & Vadaparampil ST (2019). Current approaches to cancer genetic counseling services for Spanish-Speaking patients. J Immigr Minor Healt, 21(2), 434–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle T, & McPadden E (2004). Breast cancer presents at an earlier age in Mexican American women. Breast, 10(5), 462–464. [DOI] [PubMed] [Google Scholar]
- Bradbury AR, Patrick‐Miller L, Egleston BL, Olopade OI, Daly MB, Moore CW, … Feigon M (2012). When parents disclose BRCA1/2 test results: their communication and perceptions of offspring response. Cancer, 118(13), 3417–3425. https://onlinelibrary.wiley.com/doi/pdf/10.1002/cncr.26471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes K, Linn AJ, Butow PN, & van Weert JC (2015). The characteristics and effectiveness of question prompt list interventions in oncology: a systematic review of the literature. Psycho‐Oncology, 24(3), 245–252. [DOI] [PubMed] [Google Scholar]
- Buchanan AH, Rahm AK, & Williams JL (2016). Alternate service delivery models in cancer genetic counseling: A mini-review. Front Oncol, 6, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butrick M, Kelly S, Peshkin BN, Luta G, Nusbaum R, Hooker GW, … Valdimarsdottir HB (2015). Disparities in uptake of BRCA1/2 genetic testing in a randomized trial of telephone counseling. Genet Med, 17(6), 467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarria EA, Christy SM, Simmons VN, Vadaparampil S, Gwede CK, & Meade CD (in press). Learner verification: A methodology to create suitable education materials. Health Lit Res Pract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childers CP, Childers KK, Maggard-Gibbons M, & Macinko J (2017). National estimates of genetic testing in women with a history of breast or ovarian cancer. J Clin Oncol, 35(34), 3800–3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocks K, & Torgerson DJ (2013). Sample size calculations for pilot randomized trials: a confidence interval approach. J Clin Epidemiol, 66(2), 197–201. [DOI] [PubMed] [Google Scholar]
- Cragun D, Weidner A, Lewis C, Bonner D, Kim J, Vadaparampil ST, & Pal T (2017). Racial disparities in BRCA testing and cancer risk management across a population‐based sample of young breast cancer survivors. Cancer, 123(13), 2497–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane TE, Badger TA, Sikorskii A, Segrin C, Hsu C-H, & Rosenfeld AG (2019). Trajectories of Depression and Anxiety in Latina Breast Cancer Survivors. Oncol Nurs Forum, 46(2), 217–227. [DOI] [PubMed] [Google Scholar]
- Cruz-Correa M, Perez-Mayoral J, Dutil J, Echenique M, Mosquera R, Rivera-Roman K, … Puerto Rico Clinical Cancer Genetics, C. (2017). Clinical cancer genetics disparities among Latinos. J Genet Couns, 26(3), 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czajkowski SM, Powell LH, Adler N, Naar-King S, Reynolds KD, Hunter CM, … Peterson JC (2015). From ideas to efficacy: The ORBIT model for developing behavioral treatments for chronic diseases. Health Psychology, 34(10), 971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly MB, Pilarski R, Berry M, Buys SS, Farmer M, Friedman S, … Klein C (2017). NCCN guidelines insights: genetic/familial high-risk assessment: breast and ovarian, version 2.2017. J Natl Compr Canc Ne, 15(1), 9–20. [DOI] [PubMed] [Google Scholar]
- Drohan B, Roche CA, Cusack JC, & Hughes KS (2012). Hereditary breast and ovarian cancer and other hereditary syndromes: using technology to identify carriers. Ann Surg Oncol, 19(6), 1732–1737. [DOI] [PubMed] [Google Scholar]
- Dutil J, Golubeva VA, Pacheco-Torres AL, Diaz-Zabala HJ, Matta JL, & Monteiro AN (2015). The spectrum of BRCA1 and BRCA2 alleles in Latin America and the Caribbean: A clinical perspective. Breast Cancer Res Treat, 154(3), 441–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euhus DM, & Robinson L (2013). Genetic predisposition syndromes and their management. Surgical Clinics, 93(2), 341–362. [DOI] [PubMed] [Google Scholar]
- Fejerman L, Ahmadiyeh N, Hu D, Huntsman S, Beckman KB, Caswell JL, … Carvajal-Carmona L (2014). Genome-wide association study of breast cancer in Latinas identifies novel protective variants on 6q25. Nat Commun, 5, 5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross AL, Blot WJ, & Visvanathan K (2018). BRCA1 and BRCA2 testing in medically underserved medicare beneficiaries with breast or ovarian cancer. JAMA, 320(6), 597–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwede CK, Castro E, Brandon TH, McIntyre J, Meade CD, Munoz-Antonia T, … Quinn GP (2012). Developing strategies for reducing cancer disparities via cross-institutional collaboration: outreach efforts for the partnership between the Ponce School of Medicine and the Moffitt Cancer Center. Health Promot Pract, 13(6), 807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzog MA (2008). Considerations in determining sample size for pilot studies. Res Nurs Health, 31(2), 180–191. [DOI] [PubMed] [Google Scholar]
- Hurtado-de-Mendoza A, Graves K, Gómez-Trillos S, Anderson L, Campos C, Evans C, … Sheppard V (2018). Provider’s perceptions of barriers and facilitators for Latinas to participate in genetic cancer risk assessment for hereditary breast and ovarian cancer. Healthcare, 6(3), 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado-de-Mendoza A, Jackson MC, Anderson L, & Sheppard VB (2017). The role of knowledge on genetic counseling and testing in Black cancer survivors at increased risk of carrying a BRCA1/2 mutation. J Genet Couns, 26(1), 113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp. (2017). IBM SPSS Statistics for Windows, Version 25.0 IBM Corp. [Google Scholar]
- Jagsi R, Griffith KA, Kurian AW, Morrow M, Hamilton AS, Graff JJ, … Hawley ST (2015). Concerns about cancer risk and experiences with genetic testing in a diverse population of patients with breast cancer. J Clin Oncol, 33(14), 1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John EM, Miron A, Gong G, Phipps AI, Felberg A, Li FP, … Whittemore AS (2007). Prevalence of pathogenic BRCA1 mutation carriers in 5 US racial/ethnic groups. JAMA, 298(24), 2869–2876. [DOI] [PubMed] [Google Scholar]
- Kaplan CP, Napoles A, Davis S, Lopez M, Pasick RJ, Livaudais-Toman J, & Perez-Stable EJ (2016). Latinos and cancer information: Perspectives of patients, health professionals and telephone cancer information specialists. J Health Dispar Res Pract, 9(2), 154–167. [PMC free article] [PubMed] [Google Scholar]
- Khatcheressian JL, Hurley P, Bantug E, Esserman LJ, Grunfeld E, Halberg F, … Smith TJ (2013). Breast cancer follow-up and management after primary treatment: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol, 31, 961–965. [DOI] [PubMed] [Google Scholar]
- Kinney AY, Gammon A, Coxworth J, Simonsen SE, & Arce-Laretta M (2010). Exploring attitudes, beliefs, and communication preferences of Latino community members regarding BRCA1/2 mutation testing and preventive strategies. Genet Med, 12(2), 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney AY, Steffen LE, Brumbach BH, Kohlmann W, Du R, Lee JH, … Schwartz MD (2016). Randomized noninferiority trial of telephone delivery of BRCA1/2 genetic counseling compared with in-person counseling: 1-Year follow-up. J Clin Oncol, 34(24), 2914–2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klesges LM, Estabrooks PA, Dzewaltowski DA, Bull SS, & Glasgow RE (2005). Beginning with the application in mind: designing and planning health behavior change interventions to enhance dissemination. Ann Behav Med, 29(2), 66–75. [DOI] [PubMed] [Google Scholar]
- Komenaka IK, Nodora JN, Madlensky L, Winton LM, Heberer MA, Schwab RB, … Martinez ME (2016). Participation of low-income women in genetic cancer risk assessment and BRCA 1/2 testing: The experience of a safety-net institution. J Community Genet, 7(3), 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogstad MJ, & Gonzalez-Barrera A (2015). A majority of English-speaking Hispanics in the U.S. are bilingual http://www.pewresearch.org/fact-tank/2015/03/24/a-majority-of-english-speaking-hispanics-in-the-u-s-are-bilingual/ [Google Scholar]
- Lara‐Medina F, Pérez‐Sánchez V, Saavedra‐Pérez D, Blake‐Cerda M, Arce C, Motola‐Kuba D, … Aguilar JL (2011). Triple‐negative breast cancer in Hispanic patients. Cancer, 117(16), 3658–3669. [DOI] [PubMed] [Google Scholar]
- Levy DE, Byfield SD, Comstock CB, Garber JE, Syngal S, Crown WH, & Shields AE (2011). Underutilization of BRCA1/2 testing to guide breast cancer treatment: Black and Hispanic women particularly at risk. Genet Med, 13(4), 349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindor NM, McMaster ML, Lindor CJ, & Greene MH (2008). Concise handbook of familial cancer susceptibility syndromes. JNCI monographs, 2008(38), 3–93. [DOI] [PubMed] [Google Scholar]
- Lynce F, Graves KD, Jandorf L, Ricker C, Castro E, Moreno L, … Vadaparampil ST (2016). Genomic disparities in breast cancer among Latinas. Cancer Control, 23(4), 359–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai PL, Vadaparampil ST, Breen N, McNeel TS, Wideroff L, & Graubard BI (2014). Awareness of cancer susceptibility genetic testing: the 2000, 2005, and 2010 National Health Interview Surveys. Am J Prev Med, 46(5), 440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre J, Jimenez J, Rivera YM, Sutton SK, Asencio G, Castro-Figueroa EM, … Quinn GP (2017). Comparison of health communication channels for reaching Hispanics about biobanking: A pilot trial. J Cancer Educ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda PY, Wilkinson AV, Etzel CJ, Zhou R, Jones LA, Thompson P, & Bondy ML (2011). Policy implications of early onset breast cancer among Mexican‐origin women. Cancer, 117(2), 390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network (NCCN). (2019). Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic (Version 1.2020) Retrieved April 20, 2020 from https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf [DOI] [PubMed]
- Nelson HD, Pappas M, Cantor A, Haney E, Holmes R, & Stillman L (2019). Risk assessment, genetic counseling, and genetic testing for BRCA1/2-related cancer in women: A systematic review for the US Preventive Services Task Force. JAMA, 322(7), 666–685. [DOI] [PubMed] [Google Scholar]
- Olaya W, Esquivel P, Wong JH, Morgan JW, Freeberg A, Roy-Chowdhury S, & Lum SS (2009). Disparities in BRCA testing: When insurance coverage is not a barrier. Am J Surg, 198(4), 562–565. [DOI] [PubMed] [Google Scholar]
- Quinn GP, McIntyre J, Gonzalez LE, Antonia TM, Antolino P, & Wells KJ (2013). Improving awareness of cancer clinical trials among Hispanic patients and families: Audience segmentation decisions for a media intervention. J Health Commun, 18(9), 1131–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn GP, McIntyre J, & Vadaparampil ST (2011). Preferences for hereditary breast and ovarian cancer information among Mexican, Cuban and Puerto Rican women at risk. Public Health Genomics, 14(4–5), 248–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana HQ, Cochrane SR, Hiller E, Akindele RN, Nibecker CM, Svoboda LA, … Lathan CS (2018). A comparison of cancer risk assessment and testing outcomes in patients from underserved vs. tertiary care settings. J Community Genet, 9(3), 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey-Vargas L, Sanabria-Salas MC, Fejerman L, & Serrano-Gómez S (2019). Risk factors for triple-negative breast cancer among Latina women. Cancer Epidem Biomar, 28(11), 1771–1783. [DOI] [PubMed] [Google Scholar]
- Ricker C, Lagos V, Feldman N, Hiyama S, Fuentes S, Kumar V, … MacDonald D (2006). If we build it… will they come?–establishing a cancer genetics services clinic for an underserved predominantly Latina cohort. J Genet Couns, 15(6), 505–514. [DOI] [PubMed] [Google Scholar]
- Robson M, & Offit K (2007). Management of an inherited predisposition to breast cancer. New England Journal of Medicine, 357(2), 154–162. [DOI] [PubMed] [Google Scholar]
- Rush CL, Lobo T, Serrano A, Blasini M, Campos C, & Graves KD (2016). Complementary and alternative medicine use and latina breast cancer survivors’ symptoms and functioning. Healthcare, 4(4), 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherr CL, Christie J, & Vadaparampil ST (2016). Breast cancer survivors’ knowledge of hereditary breast and ovarian cancer following genetic counseling: An exploration of general and survivor-specific knowledge items. Public Health Genom, 19(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld D (1980). Statistical considerations for pilot studies. Int J Radiat Oncol, 6(3), 371–374. [DOI] [PubMed] [Google Scholar]
- Schwartz MD, Valdimarsdottir HB, Peshkin BN, Mandelblatt J, Nusbaum R, Huang AT, … King L (2014). Randomized noninferiority trial of telephone versus in-person genetic counseling for hereditary breast and ovarian cancer. J Clin Oncol, 32(7), 618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll K, Kubendran S, & Cohen SA (2018). The past, present and future of service delivery in genetic counseling: Keeping up in the era of precision medicine. Am J Med Genet C Semin Med Genet, 178(1), 24–37. [DOI] [PubMed] [Google Scholar]
- Vadaparampil ST, Quinn GP, Dutil J, Puig M, Malo TL, McIntyre J, … Closser Z (2011). A pilot study of knowledge and interest of genetic counseling and testing for hereditary breast and ovarian cancer syndrome among Puerto Rican women. J Community Genet, 2(4), 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzel JN, Clague J, Martir-Negron A, Ogaz R, Herzog J, Jungbluth C, … Saldivar JS (2013). Prevalence and type of BRCA mutations in Hispanics undergoing genetic cancer risk assessment in the southwestern United States: a report from the Clinical Cancer Genetics Community Research Network. J Clin Oncol, 31(2), 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodson AH, Profato JL, Rizvi SH, Elsayegh N, Rieber AG, & Arun BK (2015). Service delivery model and experiences in a cancer genetics clinic for an underserved population. J Health Care Poor Underserved, 26(3), 784–791. [DOI] [PubMed] [Google Scholar]
- Yanez B, Maggard Gibbons M, Moreno PI, Jorge A, & Stanton AL (2016). Predictors of psychological outcomes in a longitudinal study of Latina breast cancer survivors. Psychol Health, 31(11), 1359–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond AS, & Snaith RP (1983). The hospital anxiety and depression scale. Acta Psychiatr Scand, 67(6), 361–370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


