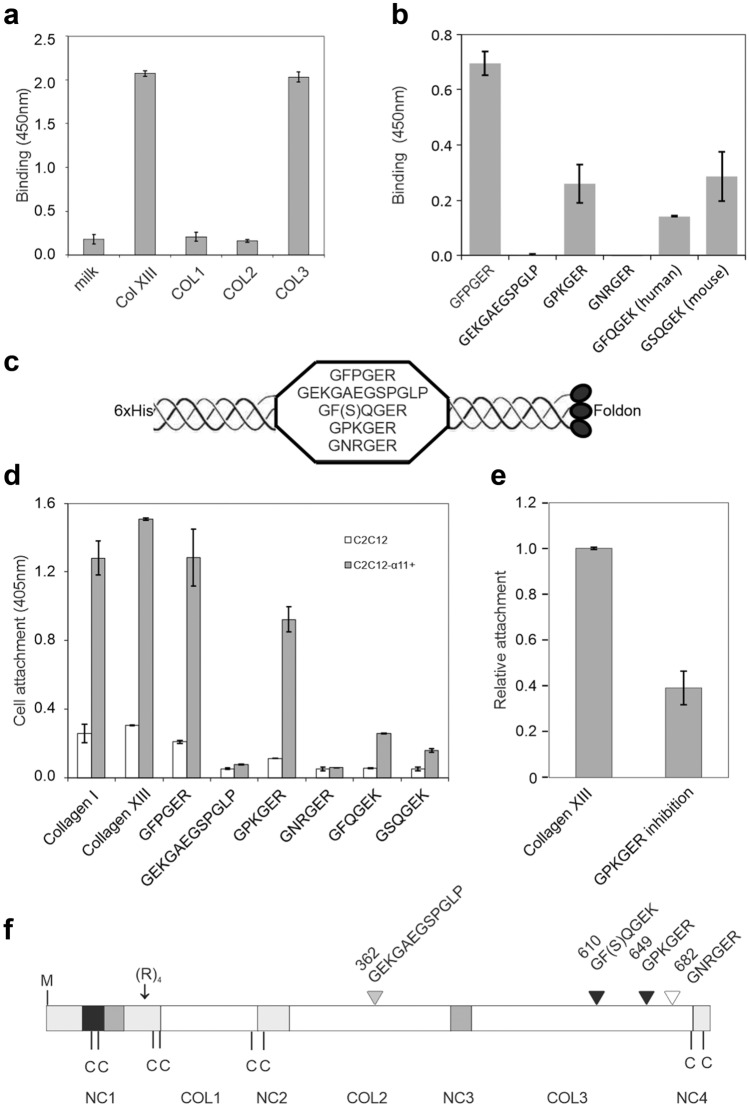
Fig. 4.
Integrin α11 binds to the COL3 domain of collagen XIII specifically by GPKGER and GF(S)QGEK. a A solid-phase binding assay shows binding of the integrin α11-I domain to the COL3 domain specifically. One hundred nanomolars of the collagen XIII ectodomain (Col XIII) and its pepsin-digested collagenous fragments, COL1, COL2, and COL3 in 50 μl of HEPES buffer were coated onto a microtiter plate wells, and 700 nM of the GST-α11-I domain in 50 μl of HEPES buffer containing 2 mM MgCl2 was added for binding testing. The bound I domain was detected by a GST antibody. b A solid-phase binding assay shows binding of the α11-I domain to the recombinant proteins composed of the testing motifs flanked by the collagenous sequence 5x(GPP) and a foldon sequence at the C-termini. c, d A cell attachment assay indicates the binding of the specific collagen XIII motifs to the integrin α11. GFPGER was used as positive control. e Cell attachment inhibition by the GPKGER motif. f A schematic structure of collagen XIII compiled from previous work (Hägg et al. 1998). The black arrowheads indicate the positive binding sites and the white shows a negative binding site. The grey arrowhead points to a reported integrin α1-binding site (Dennis J. et al. 2010). The numbers above the arrowheads represent the residues in the human collagen XIII sequence. The collagenous domains (COL1-3) are indicated as white boxes, the non-collagenous domains (NC1-4) as light gray boxes, coiled-coil motifs as dark gray boxes, and the transmembrane domain as a black box. The initiation methionine is marked as M, cysteine residues as C, and a furin proteolytic cleavage site consisting of four arginine residues as (R)4