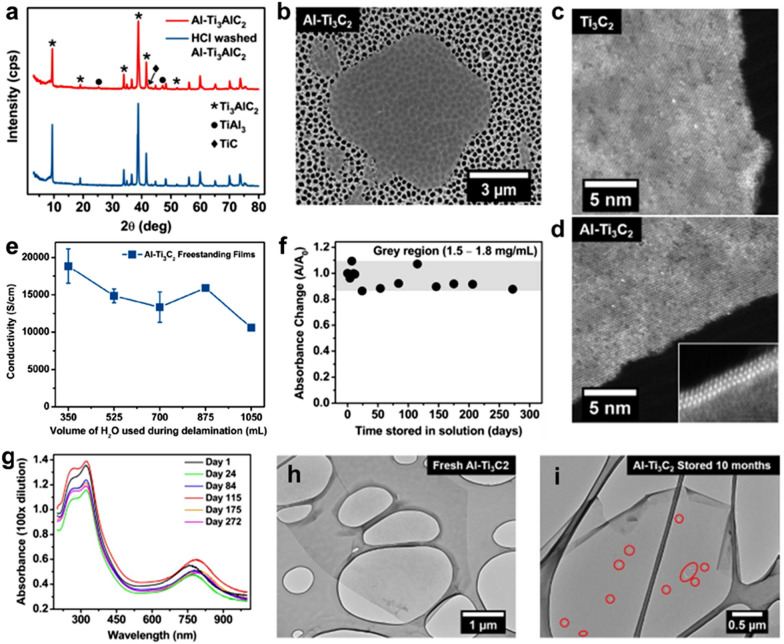
Fig. 4.
a X-ray diffraction patterns of Al-Ti3AlC2 before (red) and after (blue) HCl washing. b SEM image of a hexagonal, single-layer flake of Al-Ti3C2 produced via HF/HCl etching and LiCl delamination. c, d High-angle annular dark-field scanning transmission electron microscopy (STEM) images of Ti3C2 flake edges produced from conventional Ti3AlC2, and Al-Ti3AlC2, respectively. e Electronic conductivity of freestanding films produced by vacuum-assisted filtration of Al-Ti3C2 suspensions at different stages of the delamination process. f Absorbance changes over time for the stored Al-Ti3C2 solution calculated from the UV–vis spectra in g. The grey region corresponds to the suspension concentrations of 1.5–1.8 mg/mL. g UV–vis spectra recorded over time for an aqueous Al-Ti3C2 solution stored in ambient conditions. The TEM images of a fresh Al-Ti3C2 flake (h) and an Al-Ti3C2 flake from a ten-month-old solution (i). The red circles mark all the observable pinholes in the flake. a–i Reproduced with permissions from ref. [31]