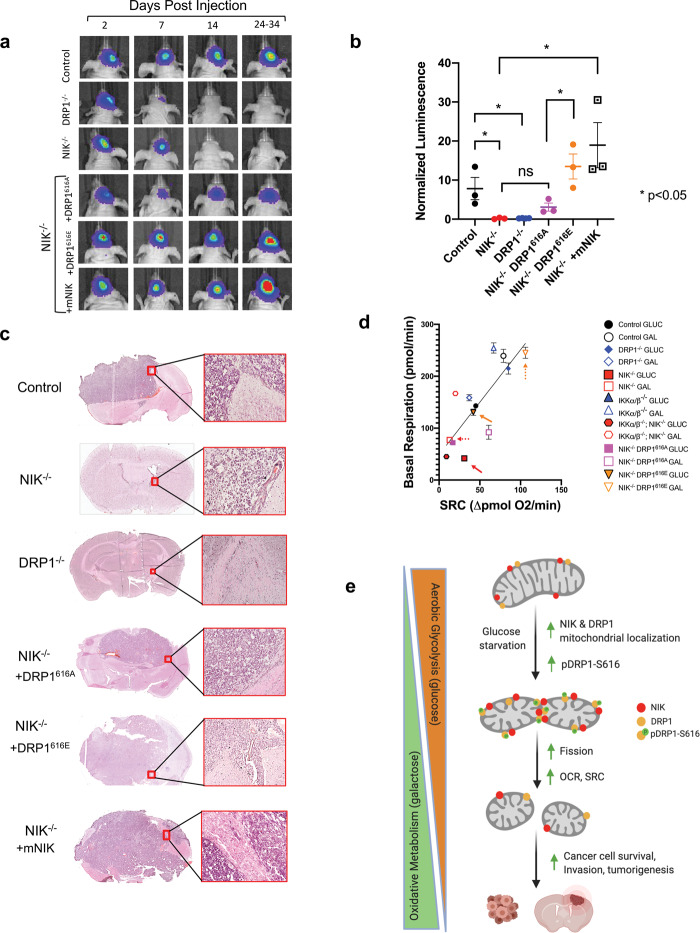
Fig. 8. Constitutively active DRP1 rescues oxidative metabolism and tumorigenic potential in NIK−/− GBM cells.
a Representative cropped images from IVIS imaging showing luminescence of individual mice pictured here over the course of 35 days, n ≥ 3 mice per cell type. Mice were imaged at regular intervals with the exception of the final endpoint since mice with large tumor growth required euthanasia at earlier timepoints. Mice harboring DRP1−/−, NIK−/−, and NIK−/−+DRP1616A tumors were last imaged at day 35 and mice harboring NIK−/−+DRP1616E and NIK−/−+DRP1616E+mNIK tumors were last imaged at days 28 and 24, respectively. b Graph depicting fold change luminescence on day 28 from fold change on day 7, with the exception of NIK−/−+mNIK which is fold change from day 21. c Representative images of tumors and histology (pictured as enlargements of red box inset) in control, NIK−/−, DRP1−/−, NIK−/− DRP1616A, NIK−/− DRP1616E, and NIK−/−+mNIK. d Composite graph plotting basal respiration vs mean spare respiratory capacity (SRC) (n ≥ 3 replicates per each cell type). Solid arrows indicate glucose condition and dotted arrows indicate galactose condition for NIK−/− and NIK−/− DRP1616E cell lines (red and orange, respectively). e Model: NIK and DRP1 mitochondrial localization increase upon glucose starvation and switch to galactose media resulting in transition from aerobic glycolysis to reliance on oxidative metabolism. NIK regulates DRP1 phosphorylation (pDRP1-S616), increases mitochondrial fission, OXPHOS, and spare respiratory capacity (SRC), leading to increased cancer cell survival, invasion, and tumorigenic potential.