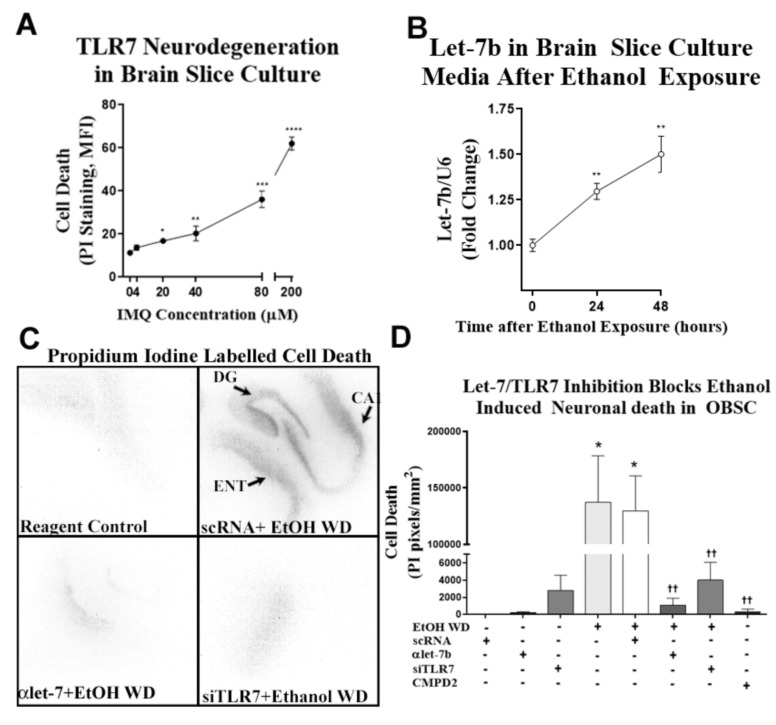
Figure 6.
Chronic ethanol causes neuronal death in mouse cortex through TLR7. (A) The TLR7 agonist imiquimod (IMQ, 72 h) induces neurodegeneration in organotypic brain slice culture (OBSC) in a concentration-dependent fashion. (B) Ex vivo OBSC was treated with ethanol (100 mM) for 4 days, followed by a 24- to 48-h ethanol-free period to induce neurodegeneration. Let-7b was measured in media microvesicles at 24 and 48 h after ethanol withdrawal. Let-7b secretion into the media increased progressively during withdrawal reaching significance at 24 h (30% increase) and at 48 h (50% increase) ** p < 0.01. (C) Representative images of propidium iodide (PI) staining showing neurodegeneration in sections containing entorhinal cortex (ENT), dentate gyrus (DG) and cornu ammonis (CA) regions of OBSC after EtOH WD. Fluorescent PI images were converted to black and white images for ease of visualization. Both the let-7 antagomir (αlet-7) and siRNA against TLR7 (siTLR7) returned PI uptake to control levels. (D) Inhibition of let-7b or TLR7 prevented ethanol-induced neurodegeneration. After ethanol treatment, media was replaced with ethanol-free media and either anti-let-7b antagomir (αlet-7b), TLR7 siRNA, or scrambled RNA (scRNA) controls. At 48 h of withdrawal, slices were assessed for cell death by PI uptake. Additional slices were treated with the TLR7 antagonist CMPD2 (50 nM) throughout ethanol exposure and withdrawal. Ethanol withdrawal (EtOH WD) caused cell death evidenced by PI uptake. The let-7b antagomir blocked EtOH WD-induced cell death, returning PI uptake to scRNA control and αlet-7b alone levels. Similarly, TLR7 inhibition by siTLR7 returned neurodegeneration to control levels. The pharmacological blockade of TLR7 with CMPD2 likewise abolished ethanol-induced cell death. * p < 0.05 vs. control, *** p < 0.001 vs. control, *** p < 0.0001 vs. control, †† p < 0.01 vs. ethanol.