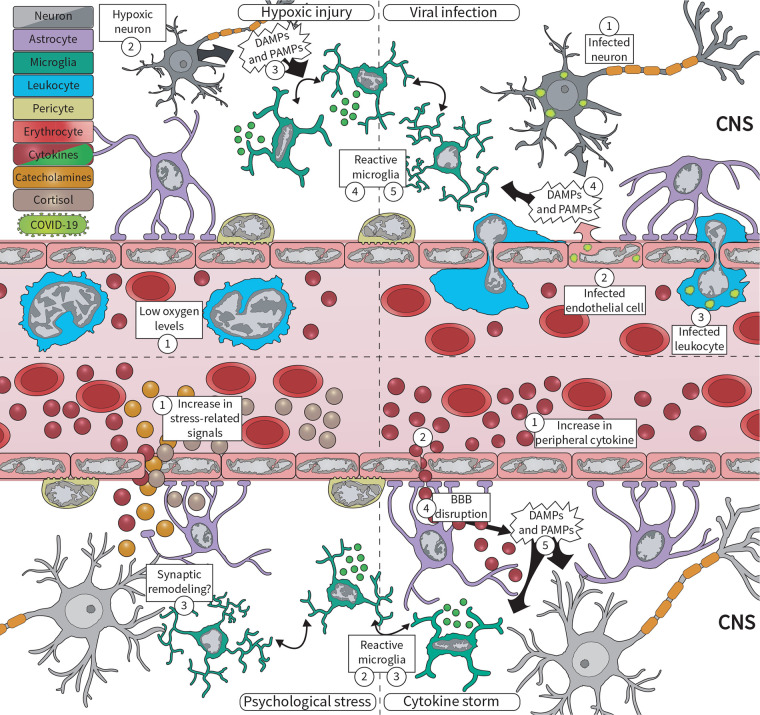
FIGURE 1.
During the COVID-19 pandemic, microglia respond to various central nervous system (CNS) insults, including viral infection, hypoxic injury, excessive circulating cytokines, and psychosocial stress. (Upper panel) Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can enter the brain via (1) peripherally infected neurons, or via (2) infected microvascular brain endothelial cells, and (3) infected infiltrating leukocytes. Infected cells, in turn, (4) release damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) that are sensed by microglia, which respond by (5) becoming reactive. At the same time, COVID-19 pathology contributes to (1) low oxygen levels, likely driving hypoxic injuries in the brain. Neurons are extremely sensitive to oxygen deficits, becoming (2) quickly damaged, and (3) releasing DAMPs and PAMPs. This is sensed by (4) microglia, which respond by changing their morphology, molecular signature, and cytokine release. (Lower panel) COVID-19 is also associated with an exacerbated inflammatory response, marked by increased levels of (1) circulating cytokines, i.e., cytokine storm. These cytokines signal to the brain via different pathways, for example, (2) signaling through the blood–brain barrier (BBB), being quickly sensed by (3) microglia. Furthermore, excessive cytokines may drive (4) BBB disruption, which increases not only cytokine levels in the brain but also of (5) DAMPs and PAMPs associated with the systemic viral infection. Lastly, psychosocial stress drives the production of cortisol, catecholamines, and cytokines, released into the (1) circulation. Microglia can respond to all of these factors by becoming (2) reactive, likely driving dysfunctional (3) synaptic remodeling.