Figure 1.
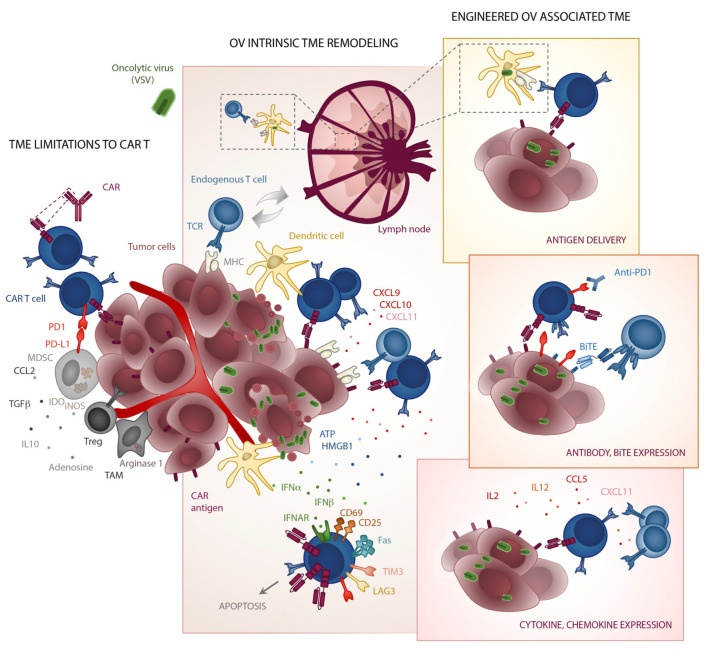
Strategic combination of oncolytic viruses with CAR T cells. The TME presents many immunosuppressive barriers to CAR T trafficking (high levels of CCL2, low levels of T cell chemotactic chemokines), as well as functionality through cytokines (TGFβ and IL10), metabolic dysregulation (arginase 1, inducible NO synthase (iNOS), indoleamine 2,3-dioxygenase (IDO), and CD39 and CD73 production of adenosine), and inhibitory ligands (PDL1 etc.). Many of these factors are expressed by tumor associated macrophages (TAMs), myeloid derived suppressor cells (MDSCs), regulatory T cells (Tregs) or the tumor cells themselves. Viral infection and oncolysis of tumor cells lead to the production of type I interferons (IFNs), danger-associated molecular pattern molecules such as HMGB1 and ATP, and CXCL9, 10, and 11 which in turn recruit additional T cells and dendritic cells. Exposure to high level type I IFN can also have inadvertent negative consequences for CAR T cells leading the upregulation of various inhibitory receptors including PD1, TIM-3 and LAG-3, as well Fas, leading to apoptosis. In contrast to some of these intrinsic properties, OVs can be armed with transgenes such as cytokines (IL2, IL12), chemokines (CCL5, CXCL11), checkpoint blocking antibodies (anti-PD1, etc.), BiTEs (EGFR, etc.) or the CAR antigen itself (CD19, etc.).