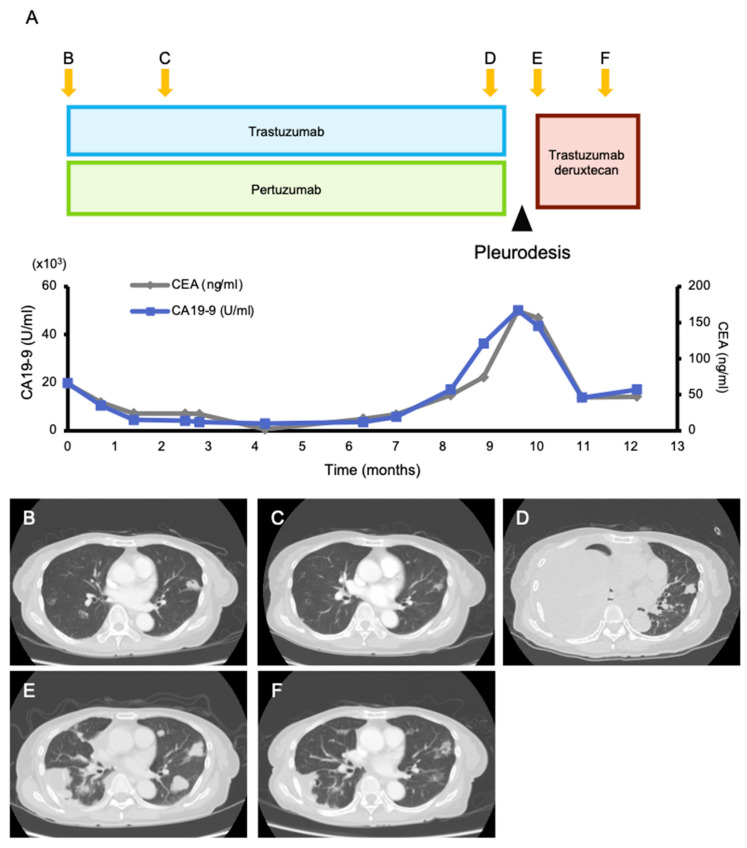
Figure 3.
Clinical presentation. (A) The course of tumor markers (carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA 19-9)) and (B–F) contrast-enhanced computed tomography (CT) images while receiving treatment with trastuzumab/pertuzumab and trastuzumab deruxtecan. Multiple lung metastases and liver metastases (which is not shown here) were observed when treatment with trastuzumab/pertuzumab was initiated (B). Two months after, a good partial response was obtained (C). After 9 months of treatment, the tumor became refractory to trastuzumab/pertuzumab, and a massive right pleural effusion was developed (D). After improvement of the pleural effusion with pleurodesis (E), the next treatment with trastuzumab deruxtecan was initiated, and tumor shrinkage was observed 1.5 months later (F).