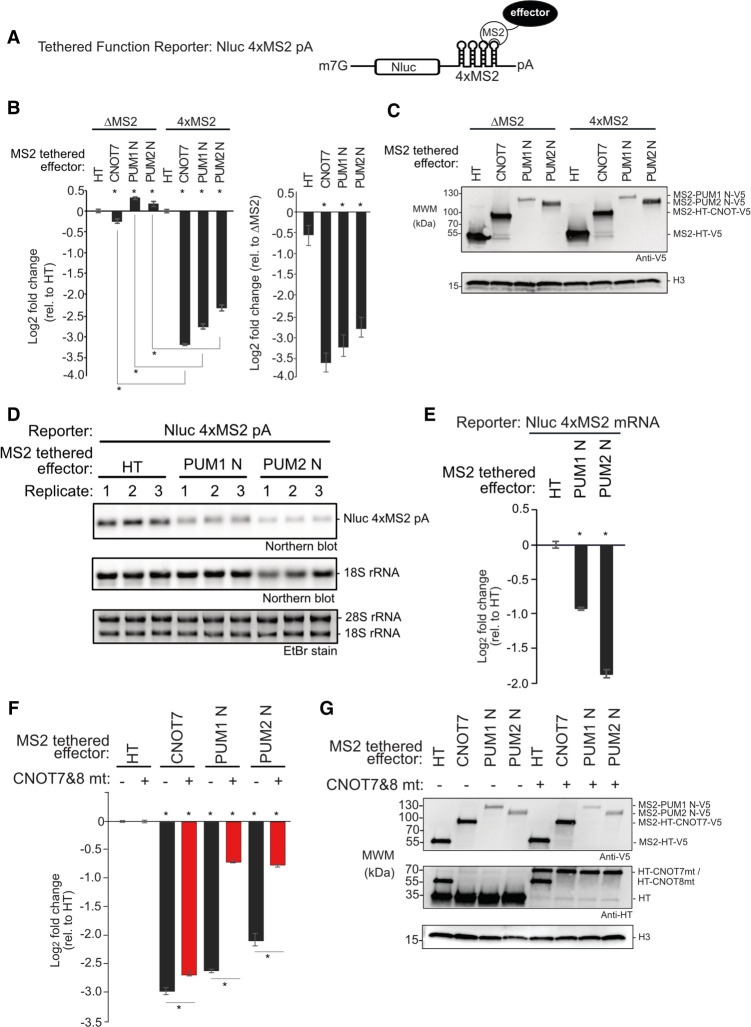
FIGURE 4.
Amino-terminal regions of human PUMs are potent repressors in isolation. Mean log2 fold change values +/- SEM are plotted and listed in Supplemental Table S1. (*) P-value < 0.05 relative to the negative control MS2-HT (above x-axis) or between the indicated conditions (below x-axis). (A) Tethered function reporter gene, Nluc 4xMS2 pA, containing four MS2 coat protein stem–loop binding sites in its 3′UTR and terminating in a 3′ poly(A) tail. (B) Repressive activity of the amino-terminal regions of PUM1&PUM2 (PUM1 N, PUM2 N) measured with the tethered function assay. PUM1&2 proteins were expressed as fusions to the RNA-binding domain of MS2 phage coat protein with a V5 epitope tag. MS2 fused to Halotag (HT) or CNOT7 served as negative and positive controls, respectively. Nluc ΔMS2 pA reporter, which lacked the MS2 binding sites, served as a negative control reporter gene. The left graph shows the activity of each effector relative to MS2-HT for each reporter, Nluc ΔMS2 pA or Nluc 4xMS2 pA. The right graph shows the activity of each effector on the Nluc 4xMS2 pA relative to the Nluc ΔMS2 pA. n = 9. (C) Western blot of proteins from a representative experimental replicate from samples in B. Histone H3 served as a loading control. (D) Northern blot of Nluc 4xMS2 pA mRNA measured the effect of tethered MS2 fusions of PUM1 N, PUM2 N, or HT. Ethidium bromide (EtBr) and northern blot detection of rRNA assessed RNA integrity and loading. n = 3. (E) Quantitation of the northern blot in D. (F) The role of CNOT in repression by tethered MS2 fusions of PUM1 N and PUM2 N was assessed using the Nluc 4xMS2 pA reporter. CNOT activity was inhibited by overexpressing HT fusions of dominant negative mutant CNOT7&8 proteins (CNOT7&8 mt, +), compared to HT (−). Repression relative to MS2-HT negative control. n = 9. (G) Western blot of V5-tagged proteins and HT-tagged CNOT7&8 mt from a representative experimental replicate from samples in F.