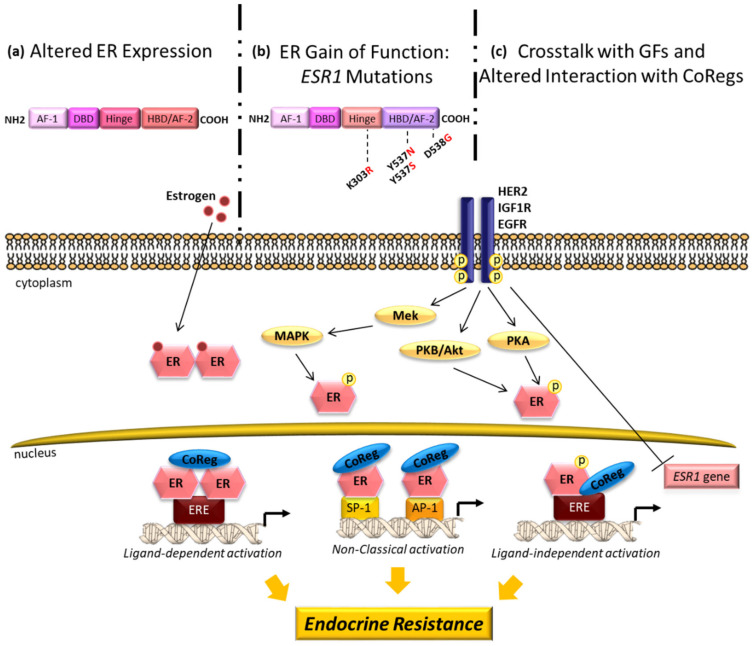
Figure 1.
Representation of the main mechanisms sustaining endocrine resistance mediated by estrogen receptor α (ERα). The structural domains of ERα contain the ligand-independent activation function (AF-1) in the amino-terminal region, a DNA-binding domain (DBD), and a carboxy-terminal hormone-binding domain (HBD), containing the ligand-dependent activation function (AF-2). In the classical ERα activation, estradiol binds to its cognate receptor in the cytoplasm, leading to dimerization, nuclear translocation and interaction with specific DNA sequences (ERE, estrogen responsive element) in target genes (ligand-dependent activation). The ERα can also bind to transcription factors such as activation protein 1 (Ap1) and specificity protein 1 (Sp1) activating gene target transcription (non-classical activation). ERα signaling activation can also occur through second messengers downstream of growth factor signaling pathways (ligand-independent activation). (a) Altered ERα expression including either loss of ERα or increased ERα expression. (b) Gain of function mutations in the ESR1 gene. The most characterized mutations within ERα were reported. The K303R somatic mutation, in the hinge domain, allows ERα to be more highly phosphorylated by Protein Kinase A (PKA) and Protein Kinase B (PKB/Akt), while the Y537N, Y537S, and D538G mutations, in the HBD/AF-2 domain, allow the receptor to be phosphorylated by Mitogen-activated protein kinase (MAPK), resulting in a ligand-independent constitutive activation of the receptor. (c) An increased bidirectional cross-talk between wild-type or mutated ERα and growth factor receptors (epidermal growth factor receptor-EGFR, the human epidermal growth factor receptor 2-HER2, the insulin-like growth factor receptor 1-IGFR 1) induces several downstream phosphorylation events that affect ERα activation, and an altered interaction of ER with coregulators affects ER transcriptional activity in a ligand-independent manner sustaining endocrine resistance. Growth factor signaling can also contribute to endocrine resistance diminishing ESR1 gene expression.