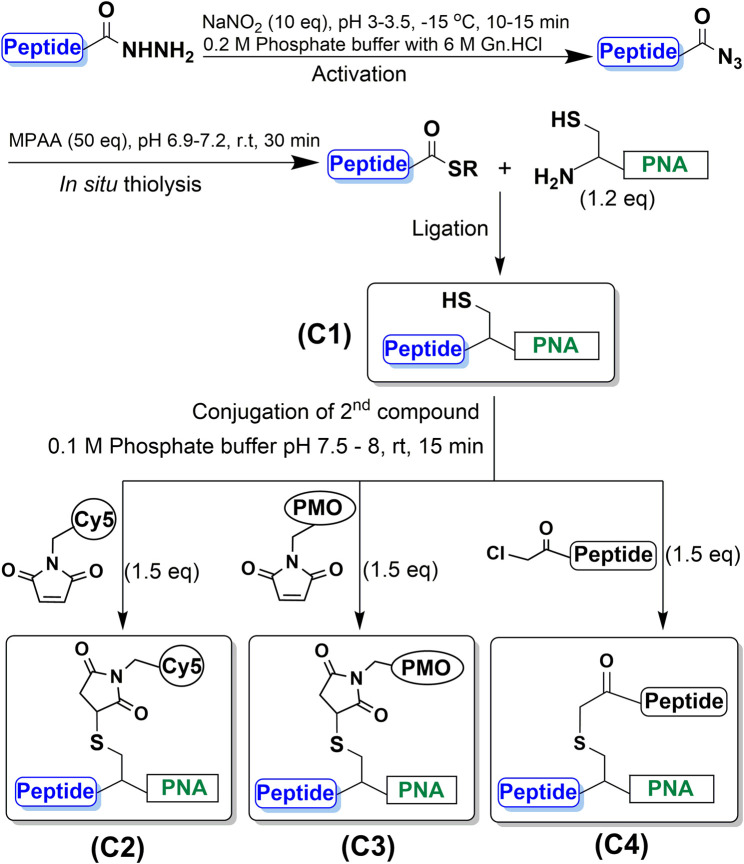
FIGURE 3.
Synthesis of CPP-PNA conjugate (C1) using native chemical ligation. Initial step of peptide-hydrazide activation using NaNO2 (10 eq) at pH 3–3.5 and −15°C for 20 min followed by in situ thiolysis to generate thioester using MPAA (50 eq) which reacts with the N-terminal cysteine to form an amide linkage. Subsequent synthesis of trifunctional conjugates (C2, C3, C4) by coupling a variety of second functional moieties to the side-chain of cysteine through thioether linkages via thiol-halide SN2 and thiol-maleimide Michael addition reactions using 1.5-fold excess of maleimide- and haloacetyl-functionalized moieties at pH 7.5–8.