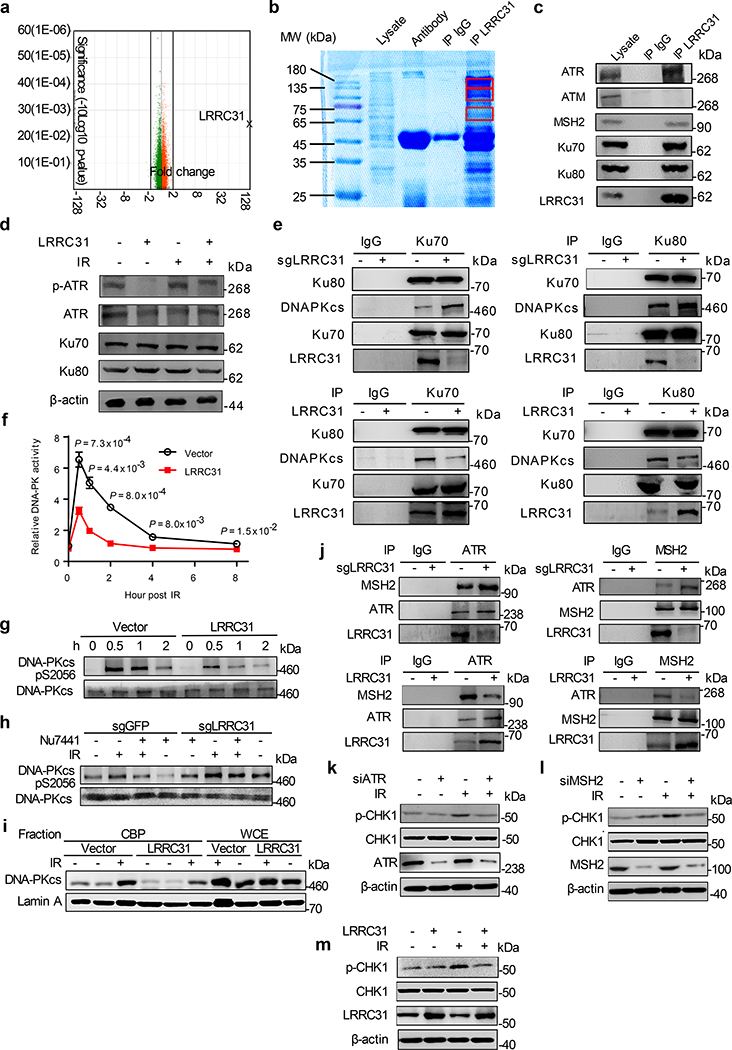
Fig. 3: LRRC31 inhibits DNA-PKcs recruitment/activation and disrupts the MSH2-ATR signaling module.
a, Whole-transcript analysis of 231BR cells with and without overexpression of LRRC31. Three biologically independent experiments were performed. Results were expressed as fold changes as a result of LRRC31 overexpression. Raw data are included in Supplementary Table 2. b, SDS-PAGE separation and Coomassie blue staining of proteins co-immunoprecipitated with a LRRC31- specific antibody. Bands indicated in the red boxes were cut and subjected to mass spectrometry analysis. c, WB analyses of proteins co-immunoprecipitated with a LRRC31- specific antibody for expression of the indicated proteins. d, WB analysis of the expression of Ku70, Ku80, ATR, and phosphorylated ATR at Ser428 (p-ATR) in cells with and without LRRC31 overexpression or/and irradiation at 4Gy. e, IP-WB analyses of the Ku70-Ku80-DNA-PKcs complex using an anti-Ku70 or an anti-Ku80 antibody. f, Changes of DNA-PK activity with time in cells with and without LRRC31 overexpression after irradiation at 4 Gy (n=3 biologically independent experiments). Data show the mean ± s.d.. Statistical analyses were performed using the two-tailed, unpaired Student’s t-test. Statistical calculations source data are included in Source Data 3. g, WB analysis of DNA-PKcs phosphorylation in cells with and without LRRC31 overexpression at the indicated time after irradiation at 4 Gy. h, WB analysis of DNA-PKcs phosphorylation in control and LRRC31 knockdown cells with indicated treatment and irradiation at 4 Gy. i, WB analysis of chromatin-recruitment of DNA-PKcs in the indicated cells after irradiation at 4 Gy. Experiments were carried out according the schematic diagram shown in Extended Data Fig 6. CBP, chromatin-banding protein; WCE, whole cell extract. j, IP-WB analyses of the formation of ATR-MSH2 complex in cells in which the expression of LRRC31 was down-regulated or up-regulated. k-m, WB analyses of the expression of CHK1 and phosphorylated CHK1 at Ser345 (p-CHK1) in cells received the indicated treatments and irradiation at 4 Gy. Blots this study are representatives of two biologically independent experiments with similar results obtained. Unprocessed immunoblots are shown in Source Data Fig. 3.