Table 1.
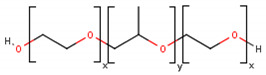
Chemical structure of some of the most commonly used polymers in hydrogels production. PEG—polyethylene glycol, PEGDA—poly (ethylene glycol) diacrylate, PLGA—poly lactic-co-glycolic acid, PVA—poly(vinyl alcohol). The oxygen (O) atoms are highlighted in red and the nitrogen (N) atoms in blue.
| Polymer | Chemical Structure |
|---|---|
| Acrylamide Polymer base for a variety of derivatives including polyacrylamide, bisacrylamide, N-Isopropylacrylamide, N-N′-dimethylacrylamide |
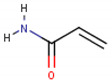
|
| Methacrylate Polymer base for a variety of derivatives including poly-2-hydroxyethylmethacrylate, poly(N,N′-dimethyl aminoethyl methacrylate), tert-butyl methacrylate, n-butyl methacrylate, n-butyl acrylate, and methyl methacrylate |
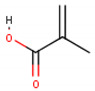
|
| PLGA (x = number of units of lactic acid and y = number of units of glycolic acid) |
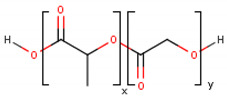
|
| PEG (n = number of units of ethylene glycol) |
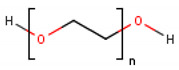
|
| PEGDA (n = number of units of ethylene glycol) |
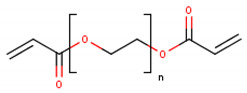
|
| Chitosan (n = number of units of β-(1→4)-linked D-glucosamine and N-acetyl-D-glucosamine) |
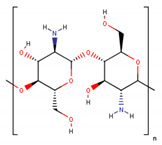
|
| Alginate (x = number of units of (1,4)-linked β-D-mannuronate and y = number of units of α-L-guluronate) |
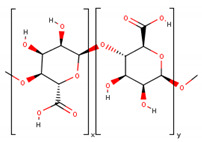
|
| PVA (n = number of units of vinyl alcohol) |
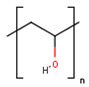
|
| Poloxamer (x = number of units of ethylene glycol and y = number of units of propylene glycol) |
|
