Abstract
Objective
Existing pharmacologic approaches for painful diabetic neuropathy (PDN) are limited in efficacy and have side effects. We examined the feasibility, acceptability, and effects of group acupuncture for PDN.
Design and Setting
We randomized patients with PDN from a public safety net hospital to 1) usual care, 2) usual care plus 12 weeks of group acupuncture once weekly, or 3) usual care plus 12 weeks of group acupuncture twice weekly.
Methods
The primary outcome was change in weekly pain intensity (daily 0–10 numerical rating scale [NRS] averaged over seven days) from baseline to week 12. We also assessed health-related quality of life and related symptoms at baseline and weeks 6, 12, and 18.
Results
We enrolled 40 patients with PDN (baseline pain = 5.3). Among participants randomized to acupuncture, 92% attended at least one treatment (mean treatments = 10.1). We observed no significant differences between once- vs twice-weekly acupuncture and combined those groups for the main analyses. Compared with usual care, participants randomized to acupuncture experienced greater decreases in pain during the 12-week intervention period (between-group differences from baseline = –2.06, 95% confidence interval [CI] = –3.01 to –1.10), but benefits were not maintained after acupuncture ended (baseline to week 18 = –0.61, 95% CI = –1.46 to 0.24). Quality of life improved for acupuncture participants (baseline to week 12 difference = 11.79, 95% CI = 1.92 to 21.66), but group differences were not significant compared with usual care (25.58, 95% CI = –3.90 to 55.06).
Conclusions
Group acupuncture is feasible and acceptable among linguistically and racially diverse safety net patients. Findings suggest clinically relevant reduction in pain from PDN and quality of life improvements associated with acupuncture, with no differences based on frequency.
Keywords: Diabetes, Pain, Acupuncture, Quality of Life, Neuropathy, Health Disparities, Randomized Clinical Trial
Introduction
Patients with diabetes and painful neuropathy commonly seek nonpharmacologic treatments [1,2], particularly when medications are ineffective or have undesired side effects. Anticonvulsants and tricyclic antidepressants provide pain relief for 25–50% of patients with painful diabetic neuropathy (PDN) but often decrease quality of life through adverse effects such as dry mouth, drowsiness, nausea, or urinary problems [3,4]. Two-thirds of patients with PDN treated with gabapentin report dizziness, somnolence, ataxia, and peripheral edema [5]. At least 20% of patients with PDN seek relief through opioid medications [6], which are often prescribed as firstline treatment [7], although evidence supporting this approach is lacking, whereas adverse effects are well known. The impact of nonpharmacologic treatments on pain management and overall quality of life among patients with PDN is understudied.
Relative to other nonpharmacologic and topical treatments of PDN symptoms, acupuncture is a promising noninvasive approach with minimal side effects [8]. Electro-acupuncture applied to rats with neuropathy resulted in improved nerve conduction velocity and increased pain threshold and nerve regeneration [9]. Small pilot studies in humans have found that acupuncture resulted in significantly improved nerve sensation and nerve conduction [10]; decreased neuropathic pain [11,12]; improved sleep, mobility, and mood [13,14]; and reduced use of pain medications in participants with PDN [14]. A meta-analysis of randomized controlled trials (RCTs) in China found that manual acupuncture improved global symptoms of PDN compared with vitamins B1 and B12 (relative risk [RR] = 1.55, 95% confidence interval [CI] = 1.33 to 1.80) and compared with no treatment (RR = 1.56, 95% CI = 1.31 to 1.85) [15,16]. Notably, trials conducted in China provided acupuncture at a greater frequency and found more robust effects associated with acupuncture than US-based studies. However, the effects of acupuncture frequency have not been formally tested.
Use of acupuncture in the United States has increased, primarily among non-Hispanic whites and those with higher education levels [17]. Racial/ethnic minorities and low-income populations, who are at greatest risk of diabetes and its related complications [18,19], often lack access to acupuncture because of financial and insurance barriers [17]. Group acupuncture—a delivery model where multiple patients simultaneously receive treatment in a common space, seated in chairs or recliners—lowers costs and improves availability [20,21], without compromising patient perceptions of quality of care [22]. Group acupuncture is a common practice in China and has been implemented in the United States for substance abuse programs [23] as part of the community acupuncture movement [20] and for chronic pain [24]. To our knowledge, group acupuncture has not been tested in diverse patients with PDN.
The rising incidence of diabetes, along with comorbidities such as chronic pain, is a growing public health concern. In this study, we sought to determine the feasibility and acceptability of group acupuncture for PDN among low-income, racially diverse patients at an urban safety net hospital. We tested the hypothesis that 12 weeks of group acupuncture would reduce average weekly pain intensity and improve health-related quality of life among patients with PDN, and we explored whether differences in the frequency of group acupuncture (once vs twice weekly) affected feasibility, treatment adherence, and clinical outcomes.
Methods
Study Design and Setting
We used a three-arm randomized, controlled, nonblinded trial design. Study participants were patients from the Zuckerberg San Francisco General Hospital and Trauma Center (ZSFG), an urban public safety net hospital that serves a diverse community of patients, of whom 70% are racial/ethnic minorities, 80% are publicly insured or uninsured, and 10% are homeless. A majority of ZSFG patients with diabetes receive care at the Richard H. Fine People’s Clinic, the Family Health Center, or Diabetes Clinic, which served as primary sites for study recruitment. At the time of the study, ZSFG hospital privileges did not yet exist for licensed acupuncturists. We therefore implemented the intervention and study visits at Community Acupuncture Works—an established clinic set up for group-based acupuncture located 0.4 miles from ZSFG.
Recruitment and Study Population
We used passive and active recruitment strategies, including posting flyers in Chinese, English, and Spanish in clinic waiting areas and outreach to ZSFG health care providers with diabetes patients. We generated a list of ZSFG patients from the San Francisco Health Network’s diabetes registry. Patients were included in the registry based on either an ICD9 250* or ICD10 E11.9, the diagnostic codes for diabetes mellitus, or two hemoglobin A1c values >6.5. We then contacted primary care physicians to confirm whether patients had diabetic neuropathy and to approve or decline eligibility of their patients for the study. Bilingual (Spanish/English and Cantonese/English) research staff were present onsite at the recruitment clinics to provide information about the study and to recruit participants. We also provided educational materials about acupuncture and incentives for study participation to bolster recruitment. We enrolled participants from March 5, 2015, through September 11, 2015.
Research coordinators assessed whether prospective participants met the following inclusion criteria for painful diabetic neuropathy: 1) diagnosed with type 2 diabetes mellitus; 2) distal lower limb pain related to neuropathy; 3) a score ≥4 on the 11-point (0–10) pain intensity numerical rating scale (PI-NRS) [25] for PDN pain; 4) pain characterized as burning, shooting, or stabbing in nature; 5) a score ≥5 on the Michigan Neuropathy Screening Instrument [26]; and 6) a score of <8 on the Semmes-Weinstein monofilament test of protective sensation [27]. Additional inclusion criteria were being aged 18 years or older; Cantonese, English, or Spanish speaking; ability to understand and willingness to comply with study procedures; and no changes in pain medications for PDN in the prior month.
Exclusion criteria were 1) acupuncture, moxibustion, cupping, or herbal medicine for PDN used within the prior two weeks; 2) electrical therapy (e.g., transcutaneous electrical nerve stimulation) or patch treatment (e.g., lidocaine or capsaicin) for PDN used within the prior two weeks; 3) pregnancy, planning a pregnancy, or breast-feeding; 4) inability or unwillingness to comply with the study protocol.
Study Procedures
Potential participants were prescreened for eligibility by telephone or in person. Research staff explained what study participation involved, answered questions about the study, and screened for basic eligibility. Potential participants were invited for an in-person screening visit at the acupuncture clinic. Once eligibility was confirmed, written informed consent was obtained from interested participants. Study materials were available in Chinese, English, and Spanish, and bilingual study staff (Cantonese/English or English/Spanish) verbally reviewed the consent form with participants.
After completing the baseline survey, we randomized participants 1:1:1 to a) usual care only, b) usual care with 12 weeks of group acupuncture once weekly, or c) usual care with 12 weeks of group acupuncture twice weekly. The data manager programmed a computer-generated table using block randomization stratified by language, with randomly selected block sizes of three and six. At the baseline visit, a research coordinator used the study database to generate a participant ID. This prompted a computerized group assignment, which was immediately recorded in the study database and could not be altered by study staff. Participants and practitioners were not blinded to group assignment, nor were interviewers, because they also were responsible for scheduling acupuncture treatments. The study data manager was blinded to participant assignment.
Participants randomized to the usual care group received gift certificates for three acupuncture treatments at the end of the study. Participants randomized to acupuncture received acupuncture treatment at no cost to them for 12 weeks. We collected data through participant-administered daily pain logs and through interview-administered surveys. All participants were provided incentives for completing interview-administered surveys at four time points ($25 at baseline, week 6, and week 12, and $35 at week 18).
Control Arm (Usual Care Only)
Usual care was provided to study participants through their primary care and/or diabetes physicians. Usual care was chosen as the control arm for this study because it is practical, feasible to implement, and clinically relevant. We considered an attention-matched control to account for the time and care intervention participants would receive through acupuncture treatments. We chose a pragmatic design and opted for usual care to address our core question of whether adjunctive acupuncture can improve symptom management compared with usual treatment. This comparison group controls for potential bias from the Hawthorne effect, regression to the mean, and the natural disease course of diabetic neuropathy during usual care.
Intervention
We developed the acupuncture study protocol using Schnyer and Allen’s manualization guidelines for flexible yet standardized treatments [28]. We conducted a systematic review of acupuncture and PDN and in-depth qualitative interviews with acupuncturists. Findings from the manualization process will be reported separately. Here we provide details of the protocol consistent with reporting standards for acupuncture clinical trials [29].
Acupuncture Rationale
We developed a standardized, reproducible protocol based on the theory and practice of traditional Chinese medicine (TCM), an acupuncture style broadly taught and practiced in the United States and China. Treatments were delivered in a group setting where patients sat in recliners and did not disrobe. The protocol therefore used points amenable to a group setting (i.e., distal points located between the elbow and fingertips, and between the knee and toe-tips) and did not use electroacupuncture, which is not typically provided in group acupuncture.
Treatment Regimen
Acupuncture treatments were administered at a community acupuncture clinic. Four study acupuncturists, each licensed for at least five years and experienced with group-based acupuncture, provided treatments over the course of the study. Two acupuncturists had conversational Spanish language skills. Bilingual study coordinators were available as interpreters when patients and acupuncturists were not language concordant. Patients were treated in the same room, but appointments were staggered, such that one patient already had needles in place when a new patient was consulting with the acupuncturist. Initial appointments were an hour in duration to allow time for individual diagnostic intake by the acupuncturist. Follow-up appointments were staggered 15 minutes apart, with a maximum of three to four patients treated per hour. Duration of follow-up assessment and needle placement was 10–15 minutes, and for needle retention it was 20–40 minutes.
Details of Needling
Acupuncture points were selected based on the anatomical location of neuropathic symptoms and participants’ individual TCM diagnoses. Eight to 12 needles were administered per treatment. Points based on anatomical location included the most distal point (jing well) and the point located near the ankle joint (shu stream) of each affected channel. Points were also selected to address individuals’ TCM diagnoses associated with the root of their PDN. For instance, SP10 was chosen if a patient presented with severe stabbing pain, indicating a TCM diagnosis of blood stagnation; LU7 and KI6 were chosen if a patient presented with dull, lingering pain, indicating a TCM diagnosis of Yin deficiency (see Supplementary Table 1 for a complete list of acupuncture points). Acupuncture was administered using sterile, disposable, surgical stainless steel needles (0.18×40 mm or 0.30×15 mm DBC Spring Ten Acupuncture Needles) inserted to standard depths recommended for each point [30]. After insertion, acupuncturists twirled the needle to achieve “de qi,” a sensation characterized as numbness or distension [30]. For each visit, study acupuncturists completed forms with details about TCM diagnosis, points used, and lengths of treatment. The principal investigator (MTC) reviewed these forms and conducted quarterly interviews with the acupuncturists to assess fidelity of intervention delivery. Acupuncturists did not deviate from the point selection, techniques, or lengths of treatment described in the study protocol.
Other Components of Treatment
Other interventions sometimes administered as part of TCM, such as moxibustion, cupping, herbs, and dietary recommendations, were not included as per the study protocol. Concomitant medications taken for symptom management of PDN, such as antidepressants, opiates, and anticonvulsants [31], were permitted as prescribed by participants’ primary care physicians. We counseled participants not to initiate the following treatments during the study: electrical spinal cord stimulation, transcutaneous electrical nerve stimulation, laser therapy, patch therapy, or other acupuncture treatment.
Outcome Measures
Using guidelines for pilot studies [32], we defined feasibility parameters, operational metrics, and goals before study implementation. The feasibility of randomization was defined as <10% of eligible patients refusing to enroll because of randomization. Our recruitment goal was to enroll 48 participants in 12 weeks. Our retention goal was ≥75% of randomized participants completing at least one in-person follow-up assessment at week 6, 12, or 18. Our adherence goal was defined as 75% of retained participants attending at least 50% of assigned treatments. We assessed acceptability of the intervention by asking participants whether they agreed or disagreed with a series of statements about their experience with acupuncture during the study, such as “If acupuncture services were offered again, I would be interested in coming for treatments.”
To test the hypothesis that group acupuncture treatment decreases pain and improves health-related quality of life among patients with PDN compared with usual care alone, we assessed core outcomes recommended for trials of chronic pain [33]. The primary outcome for the study was change in average weekly pain intensity from baseline to week 12, measured on a numerical rating scale (NRS) ranging from 0 (no pain) to 10 (worst possible pain) [34]. Participants were asked to complete a daily pain intensity log on paper or via text message from baseline to week 18. Pain levels were recorded every morning via three questions using a 24-hour recall period: average pain, worst pain, and least pain. Participants received daily text reminders to complete the log. Research coordinators collected logs from the participants on a weekly basis.
We collected data for other outcomes using the following validated surveys: the Norfolk Quality of Life Questionnaire–Diabetic Neuropathy (QOL-DN), a 35-item scale with measures of activities in the past four weeks, neuropathic symptoms, and physical and emotional health [35]; and the National Institutes of Health PROMIS forms for depression, anxiety, and sleep disturbance [36]. These questionnaires were interviewer-administered at four time points: baseline, midpoint of intervention (week 6), end of intervention (week 12), and six weeks after intervention (week 18).
We collected participants’ descriptive data during the baseline visit, including sociodemographic and additional clinical characteristics (length of time with neuropathy and sites with sensation based on monofilament testing).
Sample Size and Statistical Analysis
Using Cocks and Torgenson’s method for determining pilot sample sizes [37], we estimated that a minimum of 12 participants per group would yield sufficient power to detect an effect size between each acupuncture arm and the usual care group within an 80% confidence interval of clinically meaningful change (two-point reduction or 30% decrease in pain on the NRS) [25]. We estimated attrition of 25% and therefore sought to enroll 48 participants to retain 36 participants in the study. We assessed feasibility and acceptability by computing percentages of retention and adherence rates by group assignment.
For the three pain intensity outcomes (average, worst, and least pain level), we calculated weekly pain NRS by averaging daily pain NRS over seven days. Weekly pain levels from baseline to week 18 were analyzed based on intent to treat with linear mixed models (LMMs) including random intercepts for participants. LMMs allow modeling of all available outcome data and assume that outcome values are missing at random (MAR) conditional on observed data values [38]. For pain intensity outcomes, we compared LMMs testing linear and quadratic effects of time. We also tested whether disaggregating the acupuncture groups improved model fit: a two-group model combining the two acupuncture groups vs a three-group model distinguishing acupuncture once vs twice weekly. We chose the two-group model because it did not compromise fit based on Akaike information criteria [39]. Final LMMs for pain outcomes included fixed effects of treatment group (usual care vs acupuncture), linear time, quadratic time, and interactions between group and time effects. Additional comparisons tested simple effects of groups and time. We report data from the two-group models as our main analyses; data from the three-group comparisons are included in Supplementary Data.
To estimate the effects of acupuncture on quality of life and other patient-reported outcomes, we used an intent-to-treat analysis with LMMs including random intercepts for participants. For each outcome, we compared two models, fitting the four time points of assessment (baseline, weeks 6, 12, and 18) as either a categorical or continuous variable. We also tested whether disaggregating the acupuncture groups improved model fit. Again, we chose the two-group model because it did not compromise fit [39]. Final LMMs for quality of life outcomes included group (usual care vs acupuncture), time as a categorical variable, and group–time interaction as fixed effects. Additional comparisons tested simple effects of groups and time.
Results
Study Participants
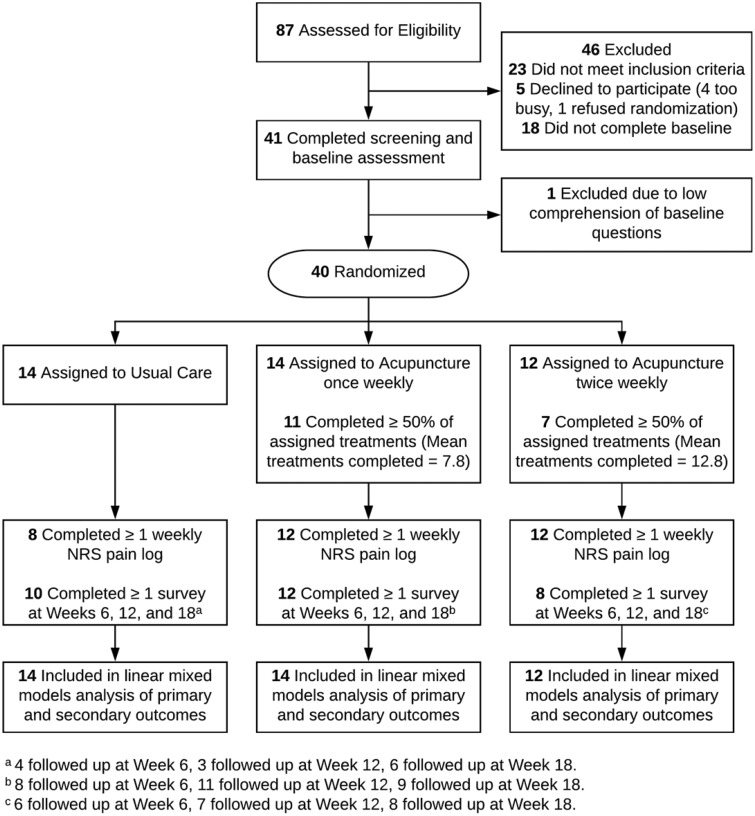
We assessed 87 ZSFG patients for eligibility and randomized 40 participants: 14 to usual care, 14 to group acupuncture once weekly, and 12 to group acupuncture twice weekly (Figure 1). Study participants had an average age of 59 (27–85) years and were 50% Latino, 20% African American, 18% non-Latino white, and 10% Asian/Pacific Islander; 93% had an annual household income <$35,000. Primary languages were English (50%) and Spanish (38%). Randomization achieved groups that were balanced in regard to important participant characteristics (Table 1).
Figure 1.
Participant flowchart.
Table 1.
Baseline characteristics of study participants
| Group Acupuncture |
|||
|---|---|---|---|
| Characteristic | Usual Care (N = 14), No. (%) | Once Weekly (N = 14), No. (%) | Twice Weekly (N = 12), No. (%) |
| Sociodemographic characteristics | |||
| Age, y* | 60.7 ± 11.8 | 61.0 ± 9.8 | 55.4 ± 11.1 |
| Sex, female | 6 (42.9) | 7 (50.0) | 7 (58.3) |
| Race/ethnicity | |||
| African American/black | 2 (14.3) | 3 (21.4) | 3 (25.0) |
| Asian/Pacific Islander | 3 (21.4) | — | 1 (8.3) |
| Latino | 7 (50.0) | 7 (50.0) | 6 (50.0) |
| Non-Latino white | 1 (7.1) | 4 (28.6) | 2 (16.7) |
| Other | 1 (7.1) | — | — |
| Nativity, born outside the US | 9 (64.3) | 8 (57.1) | 6 (50.0) |
| Educational attainment | |||
| High school or less | 7 (50.0) | 6 (42.8) | 4 (32.3) |
| Some college | 4 (28.6) | 3 (21.4) | 3 (25.0) |
| College grad or more | 3 (21.4) | 5 (35.7) | 5 (41.7) |
| Primary language | |||
| English | 6 (42.9) | 7 (50.0) | 7 (58.3) |
| Spanish | 6 (42.9) | 5 (35.7) | 4 (33.0) |
| Other | 2 (14.3) | 2 (15.3) | 1 (3.9) |
| Publicly insured | 12 (85.7) | 10 (76.9) | 11 (80.8) |
| Employment status | |||
| Employed | 5 (35.7) | 3 (23.0) | 1 (8.3) |
| Unemployed or on disability | 6 (42.9) | 3 (30.8) | 7 (58.3) |
| Retired | 3 (21.4) | 6 (46.2) | 4 (33.3) |
| Household income <$35k | 14 (100.0) | 11 (78.6) | 12 (100.0) |
| Patient-reported symptoms | |||
| Pain intensity | |||
| Average pain* | 4.4 ± 3.4 | 5.9 ± 2.3 | 5.2 ± 2.5 |
| Worst pain* | 6.4 ± 3.3 | 6.6 ± 2.5 | 6.2 ± 2.3 |
| Least pain* | 4.4 ± 3.0 | 4.9 ± 1.7 | 2.7 ± 2.9 |
| Quality of life*,† | 64.2 ± 29.7 | 71.6 ± 33.8 | 67.1 ± 31.7 |
| Physical functioning*,† | 21.7 ± 14.7 | 25.9 ± 16.6 | 22.4 ± 15.2 |
| Neuropathic symptoms*,† | 17.5 ± 6.8 | 15.9 ± 8.9 | 16 ± 7 |
| Anxiety* | 60.8 ± 9.1 | 59.0 ± 10.7 | 61.5 ± 11.7 |
| Depressive symptoms* | 57.5 ± 8.2 | 54.8 ± 12.3 | 59.7 ± 12.5 |
| Sleep disturbance* | 48.0 ± 9.8 | 45.6 ± 13.9 | 51.3 ± 14.3 |
Data presented as mean ± SD.
Norfolk Quality of Life Questionnaire–Diabetic Neuropathy.
Participants had PDN for an average of 6.3 years with moderate levels of average pain intensity (mean NRS = 5.3) in the past 24 hours at baseline; 70% reported fair or poor health status. Quality of life, presence and severity of neuropathic symptoms, and number of primary care visits were comparable between the randomized groups (Table 1; Supplementary Table 2). More participants in the once-weekly acupuncture group had changes in medications during the intervention period, compared with the other two groups (P = 0.047).
Study Feasibility
Retention was defined as completing at least one follow-up assessment. We retained 75% of participants in the study, with differences in assessment of participation by group. Fewer usual care participants completed one or more follow-up surveys at weeks 6, 12, or 18 (71% usual care vs 86% once-weekly acupuncture and 75% twice-weekly acupuncture). For weekly pain NRS logs, 57% of the usual care group completed at least one log, compared with 86% of the once-weekly acupuncture group and 100% of the twice-weekly acupuncture group.
Among participants randomized to acupuncture once weekly, 12 (86%) attended at least one treatment; 11 (79%) attended half of the assigned treatments (average = 7.8 treatments). Among participants randomized to acupuncture twice weekly, 12 (100%) attended at least one treatment; seven (58%) attended half of the assigned treatments (average = 12.8 treatments).
Acceptability of Acupuncture
All intervention participants agreed that if acupuncture services were offered again, they would be interested in coming for treatments; 82% would recommend acupuncture to others with neuropathy (80% of once-weekly participants vs 86% of twice-weekly participants); and 77% felt that they were better able to cope with neuropathy as a result of acupuncture (80% of once-weekly participants vs 71% of twice-weekly participants).
Acupuncturists completed a case form for adverse effects after each treatment. Out of 263 treatments, 15 side effects were reported: pain (8), swelling (2), numbness (2), nausea (1), cramp (1), palpitation in left arm (1). All were mild and did not require additional intervention. No serious adverse events were reported.
Pain Intensity
We present usual care vs acupuncture as our main analyses, based on tests indicating that the two-group model was optimal. We include results of the analysis of three groups (usual care vs acupuncture once weekly vs acupuncture twice weekly) in the Supplementary Data. We present LMM predicted mean outcome levels.
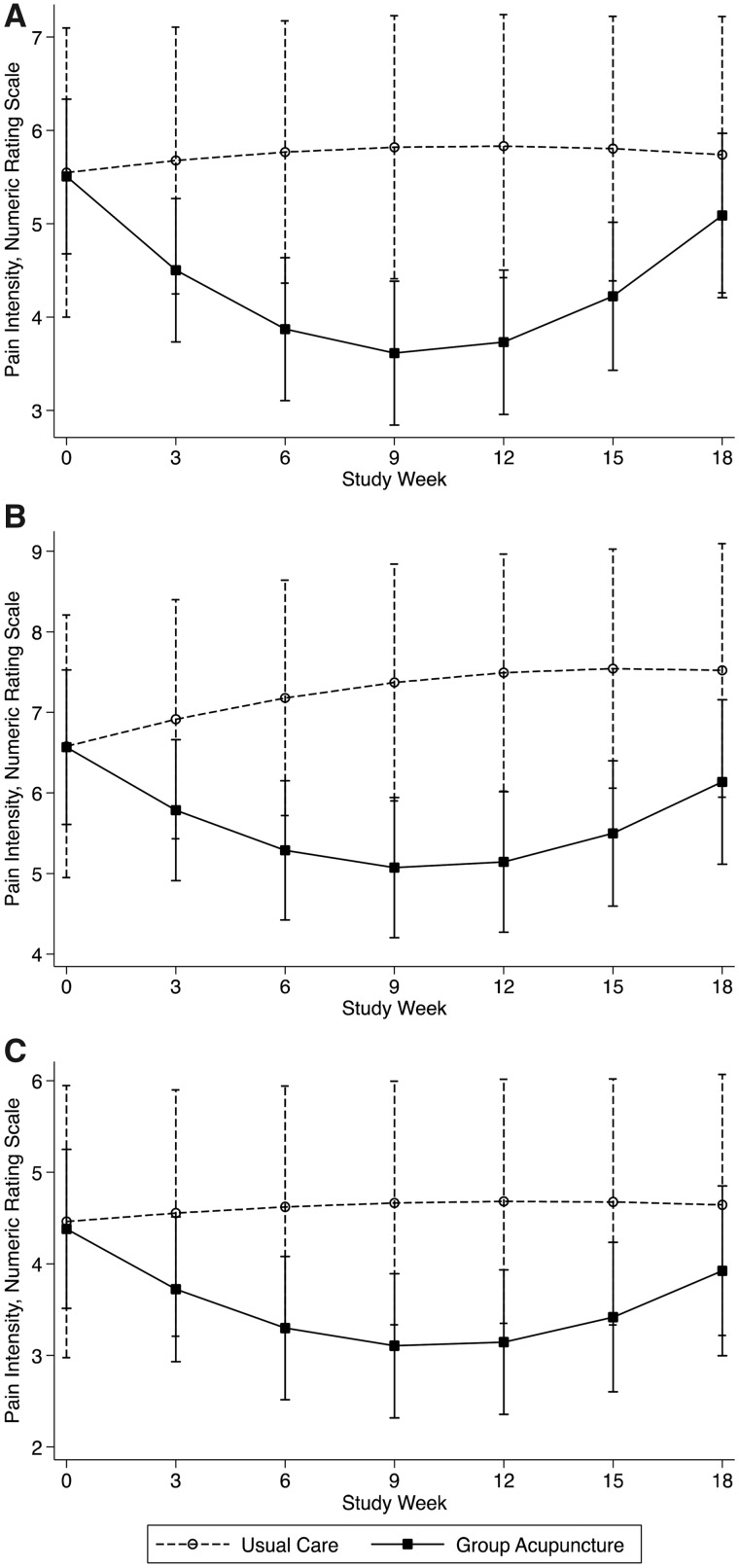
At week 12, the change in average weekly pain intensity score from baseline was significantly lower for acupuncture compared with usual care (between-group difference = –2.06 points, 95% CI = –3.01 to –1.10, P < 0.001) (Table 2). The group-by-quadratic time interaction was statistically significant (z = 4.77, P < 0.001). Average pain was relatively unchanged for the usual care group across the 18-week period (5.55 at baseline, 5.83 at week 12, and 5.74 at week 18) (Figure 2). The acupuncture group showed improvement through the 12-week intervention period (average pain NRS decreased from 5.51 to 3.73, average difference = –1.78, 95% CI = –2.23 to –1.32), but pain increased toward baseline levels by week 18 (average NRS = 5.09, baseline to week 18 difference = –0.42, 95% CI = –0.89 to 0.05).
Table 2.
Change in patient-reported outcomes, difference from baseline†
| Outcome | Usual Care | Group Acupuncture | Between-Group Differences |
|---|---|---|---|
| Average pain intensity‡ | |||
| Week 6 | 0.22 (–0.46 to 0.90) | –1.64 (–2.02 to –1.25)*** | –1.86 (–2.64 to –1.08)*** |
| Week 12 | 0.28 (–0.56 to 1.12) | –1.78 (–2.23 to –1.32)*** | –2.06 (–3.01 to –1.10)*** |
| Week 18 | 0.19 (–0.52 to 0.90) | –0.42 (–0.89 to 0.05) | –0.61 (–1.46 to 0.24) |
| Worst pain intensity‡ | |||
| Week 6 | 0.60 (–0.19 to 1.39) | –1.28 (–1.75 to –0.80)*** | –1.88 (–2.80 to –0.96)*** |
| Week 12 | 0.91 (–0.06 to 1.89) | –1.42 (–1.98 to –0.86)*** | –2.34 (–3.47 to –1.21)*** |
| Week 18 | 0.94 (0.10 to 1.79) | –0.43 (–1.03 to 0.16) | –1.37 (–2.41 to –0.34)*** |
| Least pain intensity‡ | |||
| Week 6 | 0.16 (–0.58 to 0.90) | –1.08 (–1.52 to –0.65)*** | –1.24 (–2.10 to –0.39)** |
| Week 12 | 0.22 (–0.70 to 1.14) | –1.24 (–1.75 to –0.72)*** | –1.46 (–2.51 to –0.41)** |
| Week 18 | 0.18 (–0.61 to 0.97) | –0.46 (–1.00 to 0.09) | –0.64 (–1.60 to 0.32) |
| Quality of life | |||
| Week 6 | 25.75 (5.17 to 46.32)* | 13.84 (2.93 to 24.76)* | –11.90 (–35.20 to 11.39) |
| Week 12 | –13.79 (–41.56 to 13.99) | 11.79 (1.92 to 21.66)* | 25.58 (–3.90 to 55.06) |
| Week 18 | 10.29 (–10.06 to 30.64) | 14.10 (2.87 to 25.33)* | 3.81 (–19.43 to 27.05) |
| Physical functioning | |||
| Week 6 | 10.43 (–0.58 to 21.44) | 8.15 (2.26 to 14.04)** | 2.28 (–14.77 to 10.20) |
| Week 12 | –9.69 (–24.57 to 5.19) | 6.04 (0.72 to 11.37)* | 15.73 (–0.08 to 31.54) |
| Week 18 | 7.25 (–3.66 to 18.15) | 6.83 (0.77 to 12.88)* | 0.42 (–12.89 to 12.05) |
| Neuropathic symptoms‡ | |||
| Week 6 | –7.63 (–14.92 to –0.33)* | –1.65 (–5.61 to 2.32) | 5.98 (–2.32 to 14.27) |
| Week 12 | –2.18 (–10.32 to 5.95) | –3.04 (–6.65 to 0.57) | –0.86 (–9.76 to 8.05) |
| Week 18 | 0.37 (–5.91 to 6.65) | –5.65 (–9.37 to –1.92)* | –6.01 (–13.31 to 1.29) |
| Anxiety‡ | |||
| Week 6 | –0.88 (–14.49 to 12.73) | –2.77 (–10.39 to 4.84) | –1.90 (–17.49 to 13.70) |
| Week 12 | 19.02 (3.74 to 34.31)* | –5.91 (–12.88 to 1.05) | –24.94 (–41.73 to –8.14)** |
| Week 18 | 3.33 (–8.42 to 15.08) | 4.69 (–2.46 to 11.84) | 1.36 (–12.40 to 15.12) |
| Depressive symptoms‡ | |||
| Week 6 | –1.81 (–14.52 to 10.90) | 0.27 (–6.72 to 7.25) | 2.08 (–12.43 to 16.58) |
| Week 12 | 18.84 (4.61 to 33.07)** | –1.67 (–8.04 to 4.71) | –20.51 (–36.10 to –4.92)** |
| Week 18 | 5.21 (–5.76 to 16.17) | 7.21 (0.64 to 13.77)* | 2.00 (–10.78 to 14.78) |
| Sleep disturbance‡ | |||
| Week 6 | 9.22 (–7.42 to 25.86) | 19.53 (10.37 to 28.70)* | 10.31 (–8.69 to 29.31) |
| Week 12 | 23.01 (4.37 to 41.65)* | –1.95 (–10.31 to 6.42) | –24.96 (–45.39 to –4.53)* |
| Week 18 | 6.26 (–8.09 to 20.62) | 8.51 (–0.10 to 17.13) | 2.25 (–14.49 to 18.99) |
P < 0.05; **P < 0.01; ***P < 0.001.
Data derived from linear mixed models.
Lower scores indicate more optimal outcome.
Figure 2.
Pain intensity by week, usual care vs group acupuncture. Panel A: Average pain, Panel B: Worst pain, Panel C: Least pain. Graphs are based on estimates from linear mixed models of weekly pain intensity; every third week is plotted to simplify presentation.
Change in “worst pain” from baseline to week 12 favored acupuncture (between-group difference = –2.34, 95% CI = –3.47 to –1.21) (Table 2). The group-by-quadratic time interaction was statistically significant (z = 3.42, P < 0.001). Worst pain increased slightly across time for the usual care group (baseline NRS = 6.58, week 18 NRS = 7.52) (Figure 2). For the acupuncture group, worst pain declined through week 12 (baseline NRS = 6.57, week 12 NRS = 5.14), then increased toward baseline levels (week 18 NRS = 6.14).
Change in “least pain” from baseline to week 12 favored acupuncture (between-group difference = –1.46, 95% CI = –2.51 to –0.41). The group-by-quadratic time interaction was statistically significant (z = 2.69, P < 0.01). Least pain was relatively unchanged for the usual care group, ranging from 4.46 to 4.64 across 18 weeks (Figure 2). In the acupuncture group, least pain decreased from 4.38 at baseline to 3.14 at week 12, then increased to 3.92 at week 18.
Health-Related Quality of Life
Acupuncture vs usual care differences from baseline to week 12 changes were not significant for quality of life (between-group difference = 25.58, 95% CI = –3.90 to 55.06), physical functioning (15.73, 95% CI = –0.08 to 31.54), or neuropathic symptoms (–0.86, 95% CI = –9.76 to 8.05) (Table 2). Quality of life scores improved in the acupuncture group, with a significant change from baseline to week 12 (mean within-group difference = 11.79, 95% CI = 1.92 to 21.66) that was sustained at week 18 (mean difference = 14.10, 95% CI = 2.87 to 25.33) (Table 2). Physical functioning also improved in the acupuncture group from baseline to week 12 (mean within-group difference = 6.04, 95% CI = 0.72 to 11.37), with sustained improvement at week 18 (mean within-group difference = 6.83, 95% CI = 0.77 to 12.88).
From baseline to week 12, between-group differences for anxiety (–24.94, 95% CI = –41.73 to –8.14), depressive symptoms (–20.51, 95% CI = –36.10 to –4.92), and sleep disturbance (–24.96, 95% CI = –45.39 to –4.53) were significant, primarily because these symptoms worsened in the usual care group.
Discussion
We found that group-based acupuncture is feasible to implement and is acceptable to racially and linguistically diverse patients with PDN recruited from an urban safety net hospital. We observed symptom improvement in both acupuncture groups and minimal differences between the two acupuncture groups. Taken together, this suggests that once-weekly treatments are more feasible and potentially as effective for this patient population recruited from safety net primary care clinics.
We found statistically significant reductions in pain among participants while they were receiving acupuncture treatments. Decreases of two points on a 0–10 NRS or a 30% percentage change are generally regarded as clinically relevant improvements in pain intensity [25]. At the end of the 12-week intervention, the acupuncture group reported a level of pain reduction that exceeded the usual care group’s pain reduction by 2.1 points. Similarly, for worst pain, the advantage in pain reduction reported by the acupuncture group vs the usual care group was 2.3 points. This is slightly smaller than improvements noted in clinical trials of drugs such as gabapentin. One trial found a decrease of 2.5 on the same NRS [40]. However, that treatment was also associated with substantial adverse effects, including 24% of participants reporting dizziness and 23% reporting somnolence with gabapentin, compared with only 5–6% reporting these symptoms in the control group. Overall, these results suggest that steps to optimize the degree of pain relief from acupuncture warrant further testing, with the goal of achieving more clinically significant improvement.
Decreases in pain were not sustained once acupuncture treatments ended. This is in contrast to a recent study, which found that the beneficial effects of group acupuncture on chronic musculoskeletal pain lasted 16 weeks after treatments ended [24]. Kligler et al.’s study included 24 weeks of weekly acupuncture and TCM practices (e.g., lifestyle recommendations and massage techniques) that are not typically provided in group acupuncture settings. Our study included patients with neuropathic pain and tested a 12-week treatment period of manual acupuncture. Unanswered questions include whether the durability of acupuncture effects differs for neuropathic vs nociceptive pain, what the optimal length of acupuncture treatment is, and whether less restrictive point selection or integrating other TCM practices in addition to acupuncture enhances treatment effects. The effects of ongoing maintenance treatments or use of electroacupuncture [41,42] on the durability of treatment effects also warrant exploration.
Although between-group differences were not significant, we found that acupuncture was associated with within-group improvements in quality of life, including better physical functioning and decreased neuropathic symptoms. In contrast to changes in pain intensity, which did not last beyond the treatment period, improvements in quality of life appeared more durable across the 18 study weeks. Qualitative feedback from our study participants suggested that decreasing use of medications improved their quality of life, though data extracted from participants’ health records about changes in medications were mixed and did not fully support this finding. The impact of analgesic treatments on quality of life remains an important but understudied area. Trials of pharmacologic treatments for PDN underreport quality of life but have dropout rates from adverse events that range widely (e.g., 4–39% for amitriptyline, 8–21% for gabapentin, 3–70% for oxycodone) [43].
Our study has a number of limitations, including a small sample size. Challenges included slow initial recruitment and low enrollment of Cantonese-speaking patients, limiting study generalizability. Adherence was lower for participants assigned to acupuncture twice weekly (average = 13 out of 24 treatments) compared with acupuncture once weekly (average = 8 out of 12 treatments), and retention of participants in usual care was more challenging than those receiving acupuncture. Future studies would benefit from alternative data collection methods, including telephone follow-up. Interviewers were not blind to intervention group assignment, which may have biased participant reporting of secondary outcomes. Our primary outcome, pain intensity, was self-administered and therefore not subject to the same limitation.
We did not include sham acupuncture as a comparison group and cannot assess whether the observed benefits of acupuncture were due in part to nonspecific effects of treatment. We chose a pragmatic clinical trial design and usual care as a comparison group to assess the added benefits of adjunctive acupuncture beyond existing treatment options, which are often limited to pharmacologic approaches. We recruited from a public safety net hospital and conducted the study at a clinic established for group acupuncture. Although the patient experience in the clinic is representative of a growing movement of community acupuncture sites [21], the close proximity to a safety net hospital provided a convenient location that may not be generalizable to other settings.
Despite these challenges, findings from this study suggest beneficial effects of acupuncture for neuropathic pain and quality of life and a potential worsening of symptoms among patients receiving usual care. Overall, our study suggests that group acupuncture is feasible to implement in safety net settings and is acceptable to racially and linguistically diverse patients with PDN. Group acupuncture on a once-weekly basis is a promising nonpharmacologic approach for symptom management in safety net patients with PDN. Additional research is needed to determine the optimal acupuncture protocol to enhance the clinical effectiveness of group acupuncture and the maintenance of treatment effects.
Supplementary Material
Acknowledgments
The authors thank Kaylah Sterling, MEd, LAc, Efrem Korngold, OMD, LAc, Fija Reed, MS, and Zak Zibrat, MS, for their contributions in developing the acupuncture treatment protocol; the providers at Zuckerberg San Francisco General Hospital for assistance with recruiting for the study; the study participants and acupuncturists Kate Truka, LAc, Mandy Rosenberg, LAc, and especially Ninah Hofmann, LAc, and Community Acupuncture Works for their dedication and support of this research.
Supplementary Data
Supplementary data are available at Pain Medicine online.
References
- 1. Brunelli B, Gorson KC.. The use of complementary and alternative medicines by patients with peripheral neuropathy. J Neurol Sci 2004;218(1–2):59–66. [DOI] [PubMed] [Google Scholar]
- 2. Handley MA, Quan J, Chao MT, et al. Use of complementary health approaches among diverse primary care patients with type 2 diabetes and association with cardiometabolic outcomes: From the SF Bay Collaborative Research Network (SF Bay CRN). J Am Board Fam Med 2017;305:624–31. [DOI] [PubMed] [Google Scholar]
- 3. Wong MC, Chung JW, Wong TK.. Effects of treatments for symptoms of painful diabetic neuropathy: Systematic review. BMJ 2007;3357610:87.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saarto T, Wiffen P.. Antidepressants for neuropathic pain. Cochrane Database Syst Rev 2007;4:CD005454.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moore RA, Wiffen PJ, Derry S, McQuay HJ.. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev 2011;3:CD007938.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pesa J, Meyer R, Quock TP, Rattana SK, Mody SH.. Opioid utilization patterns among Medicare patients with diabetic peripheral neuropathy. Am Health Drug Benefits 2013;64:188–96. [PMC free article] [PubMed] [Google Scholar]
- 7. Patil PR, Wolfe J, Said Q, Thomas J, Martin BC.. Opioid use in the management of diabetic peripheral neuropathy (DPN) in a large commercially insured population. Clin J Pain 2015;315:414–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boulton AJ, Vinik AI, Arezzo JC, et al. Diabetic neuropathies: A statement by the American Diabetes Association. Diabetes Care 2005;284:956–62. [DOI] [PubMed] [Google Scholar]
- 9. Inoue M, Hojo T, Yano T, Katsumi Y.. The effects of electroacupuncture on peripheral nerve regeneration in rats. Acupunct Med 2003;21(1–2):9–17. [DOI] [PubMed] [Google Scholar]
- 10. Tong Y, Guo H, Han B.. Fifteen-day acupuncture treatment relieves diabetic peripheral neuropathy. J Acupunct Meridian Stud 2010;32:95–103. [DOI] [PubMed] [Google Scholar]
- 11. Ahn AC, Bennani T, Freeman R, Hamdy O, Kaptchuk TJ.. Two styles of acupuncture for treating painful diabetic neuropathy–a pilot randomised control trial. Acupunct Med 2007;25(1–2):11–7. [DOI] [PubMed] [Google Scholar]
- 12. Garrow AP, Xing M, Vere J, Verrall B, Wang L, Jude EB.. Role of acupuncture in the management of diabetic painful neuropathy (DPN): A pilot RCT. Acupunct Med 2014;323:242–9. [DOI] [PubMed] [Google Scholar]
- 13. Walker S. A nurse-led acupuncture service for painful diabetic neuropathy. J Diabetes Nurs 2001;5:59–62. [Google Scholar]
- 14. Abuaisha BB, Costanzi JB, Boulton AJ.. Acupuncture for the treatment of chronic painful peripheral diabetic neuropathy: A long-term study. Diabetes Res Clin Pract 1998;392:115–21. [DOI] [PubMed] [Google Scholar]
- 15. Chen W, Yang GY, Liu B, Manheimer E, Liu JP.. Manual acupuncture for treatment of diabetic peripheral neuropathy: A systematic review of randomized controlled trials. PLoS One 2013;89:e73764.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dimitrova A, Murchison C, Oken B.. Acupuncture for the treatment of peripheral neuropathy: A systematic review and meta-analysis. J Altern Complement Med 2017;233:164–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang Y, Lao L, Chen H, Ceballos R.. Acupuncture use among American adults: What acupuncture practitioners can learn from National Health Interview Survey 2007? Evid Based Complement Alternat Med 2012;2012:1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Adams AS, Zhang F, Mah C, et al. Race differences in long-term diabetes management in an HMO. Diabetes Care 2005;2812:2844–9. [DOI] [PubMed] [Google Scholar]
- 19. Kanaya AM, Adler N, Moffet HH, et al. Heterogeneity of diabetes outcomes among Asians and Pacific Islanders in the US: The Diabetes Study of Northern California (DISTANCE). Diabetes Care 2011;344:930–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rohleder L. The Remedy: Integrating Acupuncture into American Health Care. Portland: Working Class Acupuncture; 2006. [Google Scholar]
- 21. Chao MT, Tippens KM, Connelly E.. Utilization of group-based, community acupuncture clinics: A comparative study with a nationally representative sample of acupuncture users. J Altern Complement Med 2012;186:561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tippens KM, Chao MT, Connelly E, Locke A.. Patient perspectives on care received at community acupuncture clinics: A qualitative thematic analysis. BMC Complement Altern Med 2013;131:293.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. National Acupuncture Detoxification Association. About NADA history. Available at: https://acudetox.com/about-nada/ (accessed October 18, 2018).
- 24. Kligler B, Nielsen A, Kohrrer C, et al. Acupuncture therapy in a group setting for chronic pain. Pain Med 2018;192:393–403. [DOI] [PubMed] [Google Scholar]
- 25. Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM.. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001;942:149–58. [DOI] [PubMed] [Google Scholar]
- 26. Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA.. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care 1994;1711:1281–9. [DOI] [PubMed] [Google Scholar]
- 27. Feng Y, Schlosser FJ, Sumpio BE.. The Semmes Weinstein monofilament examination as a screening tool for diabetic peripheral neuropathy. J Vasc Surg 2009;503:675–82, 682.e1. [DOI] [PubMed] [Google Scholar]
- 28. Schnyer RN, Allen JJ.. Bridging the gap in complementary and alternative medicine research: Manualization as a means of promoting standardization and flexibility of treatment in clinical trials of acupuncture. J Altern Complement Med 2002;85:623–34. [DOI] [PubMed] [Google Scholar]
- 29. MacPherson H, Altman DG, Hammerschlag R, et al. Revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): Extending the CONSORT statement. PLoS Med 2010;76:e1000261.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Deadman P, Al-Khafaji M, Baker K.. A Manual of Acupuncture. East Sussex, UK: Journal of Chinese Medicine Publications; 1998. [Google Scholar]
- 31. Feldman EL, McCulloch DK.. Treatment of diabetic neuropathy. In: Basow DS, ed. UpToDate. Retrieved April 20, 2019, from https://www.uptodate.com/contents/treatment-of-diabetic-neuropathy [Google Scholar]
- 32. Thabane L, Ma J, Chu R, et al. A tutorial on pilot studies: The what, why and how. BMC Med Res Methodol 2010;10:1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Turk DC, Dworkin RH, Allen RR, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain 2003;1063:337–45. [DOI] [PubMed] [Google Scholar]
- 34. Puntillo KA, Neighbor ML.. Two methods of assessing pain intensity in English-speaking and Spanish-speaking emergency department patients. J Emerg Nurs 1997;236:597–601. [DOI] [PubMed] [Google Scholar]
- 35. Vinik EJ, Hayes RP, Oglesby A, et al. The development and validation of the Norfolk QOL-DN, a new measure of patients' perception of the effects of diabetes and diabetic neuropathy. Diabetes Technol Ther 2005;73:497–508. [DOI] [PubMed] [Google Scholar]
- 36. Pilkonis PA, Choi SW, Reise SP, et al. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS(R)): Depression, anxiety, and anger. Assessment 2011;183:263–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cocks K, Torgerson DJ.. Sample size calculations for pilot randomized trials: A confidence interval approach. J Clin Epidemiol 2013;662:197–201. [DOI] [PubMed] [Google Scholar]
- 38. Fitzmaurice GM, Laird NM, Ware JH.. Applied Longitudinal Analysis. Hoboken, NJ: John Wiley & Sons; 2004. [Google Scholar]
- 39. Akaike H. Likelihood of a model and information criteria. J Econom 1981;161:3–14. [Google Scholar]
- 40. Backonja M, Beydoun A, Edwards KR, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: A randomized controlled trial. JAMA 1998;28021:1831–6. [DOI] [PubMed] [Google Scholar]
- 41. Han JS. Acupuncture: Neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci 2003;261:17–22. [DOI] [PubMed] [Google Scholar]
- 42. Mao JJ, Xie SX, Farrar JT, et al. A randomised trial of electro-acupuncture for arthralgia related to aromatase inhibitor use. Eur J Cancer 2014;502:267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Waldfogel JM, Nesbit SA, Dy SM, et al. Pharmacotherapy for diabetic peripheral neuropathy pain and quality of life: A systematic review. Neurology 2017;8820:1958–67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.