We reviewed the pertinent literature on buprenorphine, including its pharmacologic properties, studies demonstrating its analgesic potential when combined with full opioid agonists, debate surrounding provider treatment recommendations, and our institution's protocol for the treatment of patients on buprenorphine for OUD.
Keywords: Buprenorphine, Suboxone, Postoperative Pain, Opioid Use Disorder, Surgery, Acute Pain
Abstract
Objective
There is no consensus on the optimal perioperative management of patients on buprenorphine (BUP) for opioid use disorder (OUD). This article will review the available literature on BUP and the analgesic efficacy of BUP combined with full mu-opioid agonists and discuss the conflicting management strategies in the context of acute pain and our institution’s protocol for the periprocedural management of BUP.
Methods
We searched published data on BUP periprocedural management from inception through March 2018 without language restrictions. Study selection included publications reporting outcomes on perioperative pain management in OUD patients maintained on BUP.
Results
Our search resulted in four case reports supporting periprocedural discontinuation of BUP and two case series, one secondary observational study, one prospective matched cohort study, and four retrospective cohort studies supporting periprocedural continuation of BUP. No clinical trials were identified.
Conclusions
Maintaining BUP perioperatively does not lead to worsened clinical outcomes. Patients can receive adequate pain control from mu-opioid agonists while maintained on BUP. Based upon available evidence, we recommend continuing BUP at a reduced dose when indicated to avoid withdrawal symptoms and to facilitate the analgesic efficacy of mu-opioid agonists administered in combination for acute postoperative pain.
Introduction
The US Department of Health and Human Services (HHS) estimates that more than 2 million Americans suffer from opioid use disorder (OUD), characterized by the maladaptive use of opioids, leading to addiction and physical and emotional impairment [1,2]. Each day, roughly 115 deaths in the United States are due to opioid-related overdoses [3]. These staggering figures have grave societal and fiscal implications, with an economic burden estimated at $78.5 billion due to substance misuse treatment, lost productivity, and health care and criminal justice costs [4]. Opioid maintenance therapy (OMT) for OUD has been shown to reduce rates of inpatient hospitalizations and lead to overall reductions in opioid-related mortality. However, premature discontinuation of OMT can lead to opioid relapse, with estimates of up to 90% relapse when OMT is discontinued prematurely [5,6].
Buprenorphine is a high-affinity, partial mu-opioid receptor agonist that is effective as maintenance therapy for individuals suffering from OUD [7–9]. In 2016, the HHS issued rulings to extensively expand the ability of eligible practitioners to use buprenorphine pharmacotherapy for OUD [10]. As more adults are maintained on buprenorphine pharmacotherapy, controversy has ensued over appropriate strategies to manage postsurgical pain in these patients. The pharmacokinetics of buprenorphine are suggested to interfere with the analgesic efficacy of full mu-opioid receptor agonists used for acute pain [11]. However, there is no high-level clinical evidence that acute pain cannot be effectively treated in patients when buprenorphine is continued perioperatively. Further, the association between perioperative OMT discontinuation and OUD relapse is not known [11,12]. This article will review the available literature on buprenorphine, including its pharmacologic properties, preclinical and clinical studies on analgesic efficacy when combined with full opioid agonists, the debate regarding provider treatment recommendations, and our institution’s protocol for the perioperative management of patients on buprenorphine for OUD as amalgamated by the available evidence.
Methods
To review the available literature on periprocedural management of buprenorphine in OUD patients, we searched for peer-reviewed clinical studies following established methodological guidelines. We organized our search by reviewing the PubMed National Center for Biotechnology Information database. We used the Medical Subject Heading Database terms buprenorphine, buprenorphine and naloxone, opioid use disorder, surgery, perioperative pain, postoperative pain, and acute pain. Our search was performed from inception through March 2018 without publication or language restrictions. The search was supplemented by utilizing the Google search engine and by reviewing reference lists from our initial search. We restricted our search to articles on the outcomes of periprocedural continuation or discontinuation of transmucosal/sublingual buprenorphine in patients with a history of OUD. We excluded articles that covered periprocedural use of buprenorphine as part of pain medication regimens. Such articles are discussed in the section Interaction Between Buprenorphine and Opioid Agonists. Our search yielded four case reports supporting periprocedural discontinuation of buprenorphine and two case series, one secondary observational study, one prospective matched cohort study, and four retrospective cohort studies supporting periprocedural continuation of buprenorphine. Search limitations were that there were no randomized control trials evaluating continuation vs discontinuation or reducing buprenorphine perioperatively. Additionally, the majority of studies supporting buprenorphine continuation were derived from the obstetric literature (Table 1).
Table 1.
Literature supporting perioperative continuation of buprenorphine
| Authors | Year | Study Type | Buprenorphine Dose | Key Findings | Limitations |
|---|---|---|---|---|---|
| Kornfeld H, Manfredi L. [63] | 2010 | Case series | 2–24 mg | Postoperative pain adequately controlled in each patient using oral or IV full-agonist opioids. | Small sample size. All but one patient received regional anesthesia/analgesia. |
| (N = 5) | |||||
| Hansen LE, Stone GL, Matson CA, et al. [47] | 2016 | Prospective matched cohort | Unknown doses of MET and BUP combined into mean MO equiv requirements (997.1 mg) | 1) No difference in surgical complications up to one year postop and comparable analgesia in pts on BUP or MET compared with controls. | Study sample combined patients on BUP or MET. Retrospective preoperative and perioperative data. |
| (N = 17) | 2) In-hosp MO equiv use higher in BUP/MET group compared with controls. | ||||
| Macintyre PE, Russell RA, Usher KA, et al. [62] | 2013 | Retrospective | Mean = 13.7 (±6.6) mg | 1) No difference in pain/adverse effects in patients on BUP vs MET overall or when patients were not given BUP or MET POD1. | In consistent time after last dose of BUP. |
| (N = 41) | 2) Increased PCA use in patients not given BUP POD1 compared with patients given BUP. | Unknown rationale for discontinuation vs continuation of BUP patients due to retrospective nature of the study. | |||
| Jones HE, O’Grady K, Dahne J, et al. [65] | 2009 | Secondary observational study | Mean = 10.9 mg | No differences in pain scores/opioid consumption in pts on BUP vs MET following vaginal delivery (pain scores low in both groups). | Obstetric study |
| (N = 18) | (95% CL = 10.2–11.7) | Cesarean section data not included. Not directly applicable to surgical population. | |||
| Range = 8–14 mg | |||||
| Jones HE, Johnson RE, Milio L. [66] | 2006 | Case series | 18 mg | BUP and MET successfully maintained peripartum. Following cesarean section, postpartum pain adequately managed with multimodal analgesia. | Obstetric study |
| (N = 2) | Small sample size. Not directly applicable to surgical population. | ||||
| Höflich AS, Langer M, Jagsch R, et al. [83] | 2012 | Retrospective cohort control | Mean = 12.77 (±5.32) mg Vaginal delivery | 1) No difference in analgesia consumption in BUP/MET group compared with controls following vaginal delivery. | Obstetric study |
| (N = 37) | Mean = 15.33 (±7.86) mg Cesarean delivery | 2) Decreased opioid consumption in BUP/MET group compared with controls following cesarean delivery. | Retrospective design. No pain score severity data available. Possible that analgesia consumption outcomes were influenced by nonstandardized treatment strategies for opioid-dependent patients. | ||
| 3) No difference in term of pain medication requirement between BUP- and MET-maintained patients. | |||||
| Meyer M, Paranya G, Keefer Norris A, et al. [67] | 2010 | Retrospective cohort control | Mean = 13.7 (±6.2) mg Vaginal delivery | 1) No difference in opioid consumption 24 hours after vaginal delivery in BUP compared with control. | Obstetric study |
| (N = 63) | Mean = 15.5 (±6.7) mg Cesarean delivery | 2) Low but significantly higher pain scores in BUP compared with control 24 hours after vaginal delivery. | Retrospective design. No comparison between pts continued on BUP vs held or given lower dose. | ||
| 3) Pain scores and opioid consumption higher in BUP vs control after cesarean delivery. | |||||
| Vilkins AL, Bagley SM, Hahn KA, et al. [48] | 2017 | Retrospective | Mean = 16.1 (±7.8) mg | No difference in preop, intraop, or postop opioid consumption in BUP- vs MET-maintained patients. | Obstetric study |
| (N = 273) | Retrospective design |
BUP = buprenorphine; IV = intravenous; MET = methadone; MO = morphine; PCA = patient-controlled analgesia; POD = postoperative day.
Buprenorphine Pharmacologic Properties
Buprenorphine, a derivative of thebaine, is a mixed opioid receptor modulator belonging to the class of opioid agonist-antagonist analgesics [13,14]. It has partial agonist activity at the mu-opioid receptor and primarily antagonist activity at the kappa opioid receptor [13–15]. Buprenorphine’s kappa receptor antagonism has been implicated in the treatment of depressive symptoms [16]. Patients with treatment-resistant depression have responded favorably to buprenorphine therapy, and drugs that selectively antagonize kappa receptors are being designed and investigated for depression and anxiety treatment [11,17]. Buprenorphine also has agonist activity at the delta receptor; however, these effects are not well understood [14]. Buprenorphine has broad half-life variability, ranging from three hours when administered intravenously to 24–60 hours when administered sublingually [18]. Compared with other opioid analgesics, it has very slow dissociation from mu-opioid receptors. Buprenorphine’s long half-life and slow receptor dissociation are both thought to contribute to its long duration of action. Buprenorphine is not readily displaced by either mu-opioid receptor agonists or antagonists, such as naloxone, due to its high binding affinity and slow mu-opioid receptor dissociation. It is recommended that a patient should show signs of opioid withdrawal before starting buprenorphine treatment for OUD; otherwise buprenorphine rapidly displaces opioid agonist–bound mu receptors, causing precipitated withdrawal [19].
Buprenorphine provides effective analgesia at low to moderate doses and is, on average, 30 times more potent than morphine [8,20]. In studies in humans, a dose of 0.4 mg per 70 kg of buprenorphine showed no ceiling effect in analgesia, whereas a ceiling effect in respiratory depression was observed [21]. The dose range tested in this study was significantly lower than doses used for OUD treatment. A pharmacokinetic study indicated that the bioavailability of sublingual buprenorphine is approximately 30% [22]. A 0.4-mg per 70-kg dose is approximately a 1.3-mg sublingual dose per 70 kg. To the best of our knowledge, an extensive dose–response relationship of the analgesic properties of buprenorphine in human beings is not available in the literature. It is unknown whether typical OUD treatment doses (up to 32 mg sublingually per day) are associated with a ceiling effect in analgesia. In animal studies, buprenorphine displays a limited effective dose range, producing a bell-shaped dose response, with doses ranging from 0.01 mg per kg to 3 mg per kg [23]. Unlike morphine and other full-agonist opioids, the agonist effects of buprenorphine do not continue to increase linearly with increasing doses. In humans, at doses above 24 mg, up to 95% of opioid receptors are occupied with minimal increase in opioid effect [24,25]. Because of its long elimination half-life and opioid agonist effects, such as euphoria and respiratory depression that plateau with increasing doses, buprenorphine misuse potential is low, and it has proven to be successful as pharmacologic therapy for the treatment of OUD [9,26,27].
Buprenorphine Formulations for Opioid Use Disorder
Buprenorphine is currently prescribed in sublingual (Subutex, Suboxone, Zubsolv), buccal (Bunavail), implantable (Probuphine), and injectable (Sublocade) formulations for the treatment of OUD.
Suboxone, Zubsolv, and Bunavail are formulations of buprenorphine combined with naloxone in a 4:1 ratio to reduce the abuse potential of buprenorphine that occurs with aberrant intravenous use. The concentration of buprenorphine in these formulations has marked euphoric effects if administered intravenously. However, as naloxone has a significantly increased effect when injected intravenously, the euphoric effects of buprenorphine would be antagonized, precipitating immediate withdrawal, therefore discouraging misuse. Zubsolv has a slightly higher bioavailability than Suboxone. Bunavail, however, has the highest bioavailability; approximately half the dose of Bunavail is required to produce the same amount of serum bioavailability as Suboxone.
In patients who have achieved successful abstinence on low-dose daily buprenorphine therapy (8 mg Subutex equivalent or less), Probuphine subdermal implants have been Food and Drug Administration approved for OUD maintenance treatment. The implants contain 80 mg of buprenorphine hydrochloride. Steady-state concentrations are reached four weeks after placement, and plasma levels are comparable to 8 mg or less of daily Subutex therapy. Probuphine provides six months of buprenorphine maintenance [19].
For patients with moderate to severe OUD who desire more convenient opioid delivery therapy, Sublocade, extended-release buprenorphine, is available. Sublocade is administered monthly by subcutaneous injection and comes in two concentrations: 100 mg/0.5 mL and 300 mg/1.5 mL. Adults considering Sublocade treatment must be on transmucosal buprenorphine at a dose equivalent of 8–24 mg for at least seven days. The recommended dosing is a 300-mg subcutaneous injection for months 1 and 2, followed by a 100-mg injection each month thereafter. The maintenance dose can be increased to 300 mg monthly if needed. The average steady-state plasma concentration for 100 mg/mL of Sublocade is similar to 24 mg of sublingual buprenorphine (3.21 ng/mL Sublocade vs 2.91 ng/mL Subutex). Steady-state concentrations are achieved four to six months after routine subcutaneous injection [28,29].
Buprenorphine Formulations for Pain Management
Buprenorphine is available in parenteral, buccal, and transdermal formulations for the treatment of pain disorders. Buprenex, the parenteral formulation of buprenorphine (0.3 mg/mL), is approved for the treatment of moderate to severe pain and can be administered intramuscularly (IM) or intravenously (IV). The analgesic effect peaks in one hour. Buprenex is typically administered at a dose of 0.3 mg IM/IV every six hours for the treatment of moderate to severe acute pain. An additional dose of up to 0.3 mg can be administered 30–60 minutes following the initial dose if pain control is inadequate [30].
Butrans is a seven-day transdermal formulation of buprenorphine approved for the treatment of chronic pain. Butrans is available in doses ranging from 5 mcg/h to 20 mcg/h [31]. The peak concentration is six hours, and the elimination half-life is 26 hours [32]. Patients who are taking less than 30 mg oral morphine equivalents daily are advised to initiate treatment at 5 mcg/h. It is recommended that the patch concentration not be up-titrated until at least 72 hours after initiation to reduce the risk of respiratory depression and allow steady-state concentrations to be reached. During Butrans dose titration, patients should use short-acting opioids as needed [31]. Butrans doses higher than 20 mcg/h have been associated with QT prolongation; however, in European studies, doses as high as 210 mcg/h have been described [33–35]. Notably, these studies used a different transdermal buprenorphine delivery system than what is available in the United States. Butrans has been shown to have similar efficacy as other opioids in the treatment of cancer and chronic noncancer pain [36,37].
Bulbuca is a buccal preparation of buprenorphine used for chronic pain. It is available in doses ranging from 75 to 900 mcg and is administered twice daily. Similar to Bunavail, its bioavailability is higher than Suboxone and Zubsolv due to a more efficient delivery system [38].
Interaction Between Buprenorphine and Opioid Agonists
Concerns about buprenorphine antagonizing the action of mu-agonists have led to the widespread practice of withholding buprenorphine before surgery. However, such concerns need to be considered in appropriate settings, especially in regards to the dose of buprenorphine used. There is evidence in both preclinical and clinical studies that administering buprenorphine and mu-agonists in conjunction and within their respective analgesic dose ranges produces an additive analgesic response.
A preclinical study by Kogel et al. [39] demonstrated the interaction between buprenorphine with mu-opioid analgesics and mu-receptor antagonists. The combination of buprenorphine with morphine, oxycodone, hydromorphone, or fentanyl in their respective analgesic dose ranges resulted in additive or synergistic effects in the tail flick test in mice. When given after the decline of the acute buprenorphine effect, both morphine and fentanyl also showed full efficacy. However, pretreatment of the mice with high doses of buprenorphine (corresponding to the declining phase of the bell-shaped curve) clearly showed an antagonistic effect against morphine, reducing the morphine analgesic effect, even at supramaximal antinociceptive doses, to the effect of buprenorphine alone [39].
Similar results of a synergistic analgesic response occurring with concurrent administration of buprenorphine and mu-receptor agonists can be found in clinical studies. The combination of oral or intravenous morphine and a basal analgesic regimen of transdermal buprenorphine has been used as effective cancer pain treatment in humans. In an open-label study by Mercadente et al. [40], 29 cancer patients treated with transdermal buprenorphine with acceptable basal analgesia presented with episodic breakthrough pain and were given intravenous morphine at a dose equivalent to 20% of their transdermal buprenorphine dose (IV morphine 4, 6, and 8 mg for buprenorphine doses of 35, 52.5, and 70 mcg/h). Ninety-eight breakthrough pain episodes (92.4%) were treated successfully, defined as a reduction in pain intensity of more than 33% within 15 minutes; 88 of these episodes (83.0%) had more than a 50% pain intensity decrease. This study demonstrates the effectiveness of additional opioid agonists in patients receiving analgesic doses of buprenorphine [40].
Beltrutti et al. [42] reported two cases of cancer patients who experienced deep and sustained pain relief after receiving small doses of intrathecal morphine and intravenous buprenorphine [41]. The combination of intrathecal morphine and intravenous buprenorphine has also been studied for perioperative analgesia. In a randomized, double-blind, placebo-controlled study, 45 American Society of Anesthesiologists physical status II and III patients undergoing hysterectomy with general anesthesia were given intrathecal morphine 4.3 µg/kg plus IV 0.9% saline, or IV buprenorphine 1.3 µg/kg plus intrathecal saline, or intrathecal morphine 4.3 µg/kg plus IV buprenorphine 1.3 µg/kg. Postoperative pain control was managed by an additional intrathecal dose of morphine for the first group and IV buprenorphine for the other two groups. The concomitant administration of intrathecal morphine and IV buprenorphine resulted in lower pain intensity than the other groups in the 12-hour postoperative period and reached statistical significance [42], suggesting that buprenorphine and morphine at these doses interacted synergistically to reduce pain more effectively than when each medication was administered alone.
Another randomized, double-blind trial studied the concurrent use of intravenous buprenorphine and morphine in adult patients undergoing abdominal surgery [43]. The study compared the analgesic effect of buprenorphine and morphine separately and in combination for postoperative pain control in patients undergoing abdominal surgery. Patients were randomized to receive one of four regimens for 12 hours: a basal buprenorphine infusion of 0.4 µg/kg/h and buprenorphine boluses of 0.15 µg/kg each; a basal morphine infusion of 10 µg/kg/h and morphine boluses of 5 µg/kg each; a basal buprenorphine infusion of 0.4 µg/kg/h and a morphine bolus of 5 µg/kg each; or a basal morphine infusion of 10 µg/kg/h and a buprenorphine bolus of 0.15 µg/kg each. Bolus doses were delivered by intravenous patient-controlled analgesia (IV-PCA). The study showed significantly lower pain scores and superior analgesic effect of buprenorphine as compared with morphine. Furthermore, buprenorphine infusions combined with IV-PCA boluses controlled postoperative pain in the first 12 postoperative hours as effectively as morphine infusions plus boluses or the combinations of buprenorphine and morphine [43]. The overall daily intravenous buprenorphine dose used amounts of approximately 1 mg per day, which is an equivalent of 3 mg of sublingual buprenorphine.
In summary, both preclinical and clinical studies demonstrate that, at analgesic doses, the combination of buprenorphine and mu-agonists elicits an additive, and possible synergistic, response. In these studies, antagonistic effects were only seen when buprenorphine was used at doses higher than analgesic doses.
Little is known about the interaction between buprenorphine and opioid agonists when buprenorphine is used at doses to treat OUD. In a recent experimental study, Athanasos et al. showed that 12 active heroin users on buprenorphine maintenance therapy (four in a 2–8-mg daily dose range, four in a 9–15-mg daily dose range, and four in a 16–22-mg dose range) experienced no antinociception effect from high doses of morphine. Although no subgroup analysis on the three different maintenance dose groups was reported, and it is unclear whether subjects maintained with different buprenorphine doses had different antinociception responses from morphine, the result in this study is consistent with the notion that patients with OUD maintained on buprenorphine may experience a poor analgesic response from opioid agonists. These results are similar to previous studies conducted with patients on chronic opioid therapy for the treatment of noncancer pain and are identical to studies where subjects were maintained on methadone for OUD [44–46]. Although the experimental models in chronic opioid patients demonstrate lower pain thresholds despite high doses of morphine, these results are not dissimilar to those seen clinically in patients on opioid maintenance therapy. As evidenced in patients on chronic methadone therapy, although pain control is more challenging due to their increased sensitivity to pain, analgesia is achievable when high enough doses of full mu-opioid agonists are administered [47,48]. Ultimately, modification of the opioid maintenance regimen of patients on buprenorphine may be needed to achieve adequate pain control [49].
Perioperative Management in Patients of Buprenorphine
Providing adequate perioperative analgesia for patients on buprenorphine for OUD poses many challenges. As these patients are on chronic opioid therapy, they are at risk of opioid tolerance, where pain control is diminished with additional opioid use and higher analgesic doses are needed to be effective [50,51]. Clinically, this can manifest as patients on chronic opioid therapy often requiring significantly higher and more frequent doses of pain medications to achieve analgesia when compared with opioid-naïve individuals [52,53]. Rapp et al. demonstrated that surgical patients on chronic opioids for OUD, cancer, and chronic noncancer pain used on average three times higher morphine equivalents in the first 24 hours after surgery when compared with matched opioid-naïve controls [53].
An interrelated yet distinct phenomenon occurring in individuals on chronic opioid therapy is opioid-induced hyperalgesia (OIH), where increased sensitivity to painful and nonpainful stimuli develops as consequence of opioid exposure [50,51]. Although both conditions occur due to opioid administration, opioid tolerance can be overcome by administering higher doses of opioids. In contrast, escalating doses of opioids in patients with OIH can potentially worsen pain perception.
The inherent difficulty in managing pain in patients on buprenorphine, combined with the pharmacologic properties of buprenorphine discussed previously, has led to conflicting views on how to optimally treat acute pain in these patients. Currently, no high-level evidence exists to support a unified pain management strategy and, consequently, providers’ opinion diverges on whether to continue buprenorphine throughout the surgical period or to taper buprenorphine to discontinuation, supplementing with low-dose opioid agonists to prevent withdrawal [51,54,55]. The Substance Abuse and Mental Health Services Administration (SAMHSA), the branch of the HHS that manages the federal approval for buprenorphine prescribers, recommends that providers consider continuing or discontinuing buprenorphine in scenarios where moderate to severe pain is anticipated [19].
Proponents for discontinuing buprenorphine contend that elevated doses of opioids would be required to effectively treat pain in order to compete with buprenorphine’s antagonistic effects. Recommendations to discontinue buprenorphine before surgery are based on its pharmacologic properties and accounts where patients had poorly controlled acute pain while maintained on buprenorphine. McCormick et al. described a 50-year-old man with McArdle’s disease and acute compartment syndrome on buprenorphine for chronic pain and opioid dependence that required discontinuation of buprenorphine and significantly higher-than-usual doses of hydromorphone in order to achieve pain relief. The authors contend that the patient’s pain was not effectively controlled until 48 hours after discontinuation of buprenorphine [56]. Similarly, Huang et al. reported the case of a 47-year-old female on buprenorphine for chronic pain who underwent a thoracotomy window closure procedure for pulmonary aspergilliosis. Her postoperative pain was ineffectively controlled despite high doses of intravenous hydromorphone. When buprenorphine was discontinued, her pain improved, and her opioid requirement was reduced [57]. Gilmore et al. described a case report of a 22-year-old male who presented to the emergency department with a comminuted distal radial and ulnar fracture caused by a work-related injury. His pain was ineffectively treated despite receiving 10 mg of morphine and a 1-µg/kg bolus of remifentanil with a remifentanil infusion at 1.7 µg/kg/min. When his providers discovered that he was on buprenorphine, opioid analgesics were discontinued, his pain was successfully treated with a Beir block, and his fracture was then able to be reduced [58]. Brummett et al. described a patient on buprenorphine who underwent a posterior spinal fusion. In order for his postoperative pain to be adequately controlled, he required discontinuation of buprenorphine and transfer to the intensive care unit for a dexmedetomidine infusion and high-dose opioid therapy [59].
These authors contend that buprenorphine should be discontinued at least 72 hours before elective surgery and replaced with opioid agonists to prevent withdrawal. However, there is evidence to support that acute pain control is challenging in patients on buprenorphine irrespective of continuation. Israel et al. described a patient who had buprenorphine discontinued three days before bilateral mastectomy. Even though her buprenorphine was held, her pain was still poorly controlled [60]. Similarly, Chern et al. described a 37-year-old woman on buprenorphine who underwent two separate urogynecologic procedures. In one procedure, the patient took buprenorphine up to the day of surgery, and for her second procedure, buprenorphine was discontinued five days before surgery and replaced with hydromorphone. Her pain was poorly controlled in both instances [61].
These cases highlight that acute pain management in patients taking buprenorphine for OUD is complex, multifactorial, and influenced by more than the pharmacologic properties associated with concomitant buprenorphine and full opioid agonist administration. Moreover, there is evidence indicating that acute pain management can be achieved comparable to methadone when buprenorphine is continued perioperatively. In a retrospective study evaluating surgical outcomes following total joint arthroplasty in patients on methadone or buprenorphine compared with matched controls, there was no difference in long-term complications up to one year following surgery. Patients on methadone or buprenorphine required an almost eight-fold higher amount of opioids in the perioperative period; however, their pain scores were not significantly different than controls [47]. In another retrospective study of a mixed surgical cohort taking buprenorphine or methadone, postoperative pain scores and patient-controlled analgesia requirements were similar on postoperative day 1 in both groups independent of surgical type [62]. Interestingly, patients whose buprenorphine was held on postoperative day 1 required significantly more opioids in morphine equivalents than those for whom buprenorphine was continued (245.5 mg +/-109.3 vs 155.2 mg +/- 135.5). There was no significant difference in opioid consumption in patients where methadone was continued vs held. In a case series where five patients were maintained on buprenorphine perioperatively, Kornfeld et al. reported that adequate pain control was achieved in all participants by using oral or intravenous opioid agonists and, in all but one patient, regional anesthesia [63]. These studies demonstrate that pain control can be managed in patients where buprenorphine is continued perioperatively with favorable clinical outcomes.
Further evidence that acute pain can be controlled in the presence of buprenorphine is found in studies conducted in pregnant women. Buprenorphine is increasingly being prescribed for OUD during pregnancy and has been shown to be safe for mother and child throughout gestation, delivery, and while breastfeeding. The Maternal Opioid Treatment: Human Experimental Research (MOTHER) study found that when compared with methadone, buprenorphine-exposed newborns experienced less severe neonatal abstinence syndrome (NAS), required 89% less morphine, needed shorter tapers, and had reduced hospital stays [64]. Not only is buprenorphine typically continued throughout pregnancy and before elective cesarean section to avoid NAS, but it is also not feasible to discontinue buprenorphine in the days before labor as childbirth is inherently unpredictable. Although the data on pain control during labor and delivery are not immediately applicable to the surgical population, evaluating acute pain management in this group provides insights into the feasibility of postsurgical pain control with buprenorphine continuation.
Jones et al. investigated pain control and analgesic requirements following vaginal delivery in parturients continued on buprenorphine or methadone. Most parturients received an epidural for labor, and all patients were treated with oxycodone, acetaminophen, and ibuprofen for pain control following delivery. Pain scores were similar in both groups (mean visual analog scale [VAS] = 4.4) and decreased over the five-day period studied, along with a reduction in opioid consumption. The study concluded that parturients on buprenorphine and methadone can have comparably effective acute pain management when multimodal analgesia is employed following vaginal delivery [65]. Two patients in the same study cohort had cesarean sections, one maintained on buprenorphine and one maintained on methadone. Both patients were able to achieve adequate pain relief when maintenance therapy was continued throughout the peripartum period [66].
In a retrospective study where parturients with history of OUD maintained on buprenorphine were compared with controls, there was no difference in opioid consumption following vaginal delivery in the first 24 hours postpartum [67]. Pain scores were measured using the VAS (0–10). Although pain scores were low in both groups (2.7 vs 2.1), they were significantly higher in the patients on buprenorphine. In parturients who required cesarean section, both pain scores (5.1 vs 3.3) and opioid consumption, measured in morphine equivalents (89.3 mg vs 60.9 mg), were significantly higher in the buprenorphine patients. The authors contended that, although opioid consumption was increased in the buprenorphine group compared with controls, buprenorphine can safely be continued during labor and delivery while maintaining adequate acute pain control. The authors also commented that their results were similar to studies where parturients on methadone exhibited increased opioid consumption and pain scores when compared with opioid-naïve patients and surmised that possible reasons for the increased opioid requirements were 1) hyperalgesia and opioid tolerance as seen in patients on chronic opioids and/or 2) a less-than-expected influence of buprenorphine receptor binding on clinical outcomes, as they expected patients in the buprenorphine group to have more pain and be less responsive to opioid analgesics. Indeed, in a recent retrospective study comparing parturients on methadone with those on buprenorphine, there were no statistically significant differences in preoperative, intraoperative, or postoperative opioid consumption [48]. These studies both conclude that buprenorphine does not need to be held for adequate acute pain control in parturients and support that continuation of buprenorphine is feasible and does not intractably interfere with postoperative analgesia. In Table 1, we summarize the above evidence supporting the continuation of buprenorphine during the perioperative period.
Further reason to continue buprenorphine perioperatively is that discontinuation of buprenorphine has been associated with increased rates of illicit opioid use [6,68–71]. In a multicenter trial of primary heroin or prescription opioid users seeking treatment for opioid dependence, 82% of the 516 participants enrolled were unable to remain abstinent from opioids one month following buprenorphine cessation [68]. Similarly, in a multicenter observational study of prescription opioid users, only 9% of study participants successfully refrained from opioid misuse, as defined by urinalysis-verified self-reports of opioid abstinence [6]. In studies where participants were started on naltrexone following buprenorphine discontinuation, rates of illicit opioid use surpassed 50% [71,72]. Considering these poor outcomes, the buprenorphine treatment duration required to prevent opioid relapse following buprenorphine discontinuation is not known [19]. Thus, it is more favorable for patients to maintain buprenorphine perioperatively as abrupt discontinuation while supplementing with opioid medications misused in the past can serve as a trigger for relapse.
Receptor Availability Studies
Receptor binding studies provide insight into the dose range where buprenorphine can have an additive effect when used together with agonists, as well as determining the optimal time interval between administration of buprenorphine and agonists so that agonist administration is coincident with opioid receptor availability. Understanding such dose and time–receptor availability correlations is extremely important for clinicians to devise optimal management of the timing and dose administration of buprenorphine during the perioperative period.
Human [11C]-carfentanil positron emission tomography (PET) studies provide direct insight into receptor occupancy at different buprenorphine doses and at different time points after administration. In a [11C]-carfentanil PET study, Zubieta et al. [25] examined in vivo mu-opioid receptor binding in three healthy opioid-dependent volunteers during maintenance on 2- and 16-mg sublingual buprenorphine and after detoxification (0 mg) under double-blind, placebo-controlled conditions. Buprenorphine induced dose-dependent reductions in mu-opioid receptor availability, 36–50% at 2 mg and 79–95% at 16 mg relative to placebo, in multiple brain regions including the prefrontal cortex, anterior cingulate, caudate, putamen, thalamus, amygdala, and cerebellum [25].
Greenwald et al. [24] further investigated the relationship between buprenorphine maintenance doses and mu-opioid receptor availability, plasma concentrations, and antagonist blockade in five heroin-dependent volunteers who were successively maintained on 32-, 16-, 2-, and 0-mg daily buprenorphine doses. Higher buprenorphine doses decreased in vivo mu-opioid receptor availability, measured with [11C]carfentanil PET scans, increased plasma levels of buprenorphine and its metabolite nor-buprenorphine, and decreased withdrawal symptoms and hydromorphone responses. Buprenorphine dose-dependently decreased mu-opioid receptor availability, increased plasma levels of buprenorphine, decreased opioid withdrawal symptoms, and attenuated opioid agonist effects. High-dose buprenorphine maintenance produced near-maximal mu-opioid receptor occupation. At the 32-mg daily dose, buprenorphine blocked 94–98% of mu-opioid receptors in most regions throughout the brain, including the prefrontal cortex, anterior cingulate, amygdala, nucleus accumbens, and caudate, with the exception of an 88% blockade in the thalamus. In a later study, the same group combined the above two PET studies and presented a curve fit of the imaging studies to extrapolate mu-opioid receptor availability in various brain regions over a 16-fold range of doses spanning from 2 mg to 32 mg daily [73,74]. They estimated the receptor availability at different daily maintenance buprenorphine doses as the following: 71–85% at 1 mg, 53–72% at 2 mg, 36–55% at 4 mg, 11–22% at 8 mg, 13–24% at 12 mg, 9–20% at 16 mg, 4–15% at 24 mg, and 2–12% at 32 mg.
Opioid receptor availability can also be estimated by mathematic simulation based on measurement of medication-intrinsic efficacy relative to placebo control that corresponds to the fraction of receptors. Comer et al. conducted a study of heroin-dependent volunteers without brain imaging that estimated relationships between receptor availability and heroin’s reinforcing and subjective effects after self-administration in a controlled experimental setting. The self-administration and subjective effects data for heroin in the presence of buprenorphine/naloxone were compared with a separate control group of eight recently detoxified participants in order to obtain estimates for the apparent in vivo dissociation constant, the efficacy estimate, and the estimated fraction of receptors remaining after buprenorphine/naloxone treatment for mathematic modeling and curve fitting. Their study showed that 2, 8, and 32 mg of buprenorphine dose-dependently reduced the receptor availability by 74%, 83%, and 91%, respectively [75].
Taken together, these receptor availability studies show that at high buprenorphine maintenance doses (24–32 mg daily), the availability of mu-opioid receptors is minimal, whereas at moderate doses (8–12 mg daily), there is still up to 20% mu-opioid receptor availability. This dose range also corresponds to buprenorphine’s analgesic dose. An open-label study in chronic pain patients reported that daily sublingual doses of buprenorphine ranging from 4 to 16 mg (mean = 8 mg) in divided doses provided satisfactory pain relief [76].
The time course of buprenorphine-induced mu-opioid receptor availability is also important for clinicians to determine the time when the buprenorphine dose should be changed in preparing patients for surgery and perioperative pain management.
Preclinical studies have shown that, although buprenorphine is associated with slow receptor dissociation in vitro, receptor binding is reversible within the duration of analgesic action in ex vivo binding studies in rats [77]. Englberger et al. showed that administration of buprenorphine (dose range from 4.64 to 46.4 μg/kg intravenously) resulted in potent, dose-dependent antinociception in the rat tail flick assay. The maximal binding capacity (Bmax) for [3H]-[D-Ala2, N-methyl-Phe4-Gly5-ol]-enkephalin ([3H] DAMGO) in isolated rat forebrain, which represents the amount of unbound opioid receptors, correlated inversely with the antinociceptive activity in the rat tail flick test (ED50 16.4 μg/kg intravenously one hour postapplication). At eight hours after administration, there was still residual antinociception, but no further attenuation of Bmax was detectable. Thus, receptor occupancy by buprenorphine does not cause impairment of μ-opioid receptor accessibility beyond the duration of its antinociceptive activity [77].
The time course for mu-opioid receptor availability, opioid withdrawal symptoms, and plasma concentration after buprenorphine cessation was reported in humans by Greenwald et al. [73]. In this study, 10 heroin-dependent volunteers stabilized on buprenorphine 16-mg/d were asked to withhold their buprenorphine. Availability of mu-opioid receptors (measured with [11C]-carfentanil PET), plasma buprenorphine concentration, opioid withdrawal symptoms, and blockade of hydromorphone effects were measured at four, 28, 52, and 76 hours after the last daily dose of buprenorphine. Receptor availability in brain regions showed highly similar time-dependent effects, ranging from 27% to 31% at four hours, 54% to 61% at 28 hours, 66% to 75% at 52 hours, and 77% to 94% at 76 hours. Whole-brain mu-opioid receptor availability was 30%, 54%, 67%, and 82% at four, 28, 52, and 76 hours after last dose of buprenorphine. The withdrawal symptoms at 28 hours did not differ from at four hours after last dose, but withdrawal symptoms at 52 hours were significantly more than at four hours, suggesting that approximately 50–60% receptor occupancy by buprenorphine is required for adequate withdrawal symptom suppression (in the absence of other opioids).
These preclinical and clinical studies demonstrate reversible binding of buprenorphine to mu-opioid receptors. Buprenorphine binding is required for suppression of opioid withdrawal, but it is still permissible for additional mu-agonists to have an analgesic effect if there is adequate receptor availability when buprenorphine is given at the appropriate dose and time. These studies provide important insight into the optimal dosing strategies of buprenorphine in the perioperative period to minimize withdrawal and permit the analgesic effects of mu-agonists when given simultaneously. It should be noted that these studies focused on the brain reward pathway. Although they provide direct evidence on the correlation between receptor binding and withdrawal prevention, caution should be taken in extrapolating the conclusions to the pain pathways. Nevertheless, there are currently no studies focusing on receptor binding in pain pathways in OUD subjects maintained on buprenorphine. On the other hand, there have been no reports so far that suggest that opioid receptor binding in the pain pathways is drastically different from that in the reward pathway. Future studies specifically addressing receptor availability in pain pathways in this patient population will enhance our understanding of opioid analgesia in this context.
Recommended Guideline for Perioperative Management of Buprenorphine
Although there are wide variations in the management of buprenorphine in the perioperative period, we feel that it is unnecessary and that it may be harmful to have patients on buprenorphine completely stop their maintenance medication before surgery. Many institutions suggest withholding buprenorphine for 48–72 hours before surgery. However, this introduces the risk of opioid withdrawal, relapse of OUD while off buprenorphine, and exacerbation of chronic pain. Patients are generally anxious and even fearful of the withdrawal reaction they may have while buprenorphine is stopped, as well as the withdrawal reaction they may have when they transition from postoperative opioid agonist pain medications back to buprenorphine. Furthermore, buprenorphine has been shown to relieve depression refractory to other forms of medical management through kappa receptor antagonism [11]; thus discontinuation could potentially worsen depressive symptoms. The evidence reviewed above suggests that analgesic or moderate doses of buprenorphine combined with opioid agonists can have an additive, instead of antagonistic, analgesic effect. Buprenorphine-maintained patients need 50–60% of opioid receptors occupied by buprenorphine to avoid withdrawal reactions in the absence of other opioid agents. Thus, it is more desirable to maintain these patients on a moderate dose of buprenorphine throughout the perioperative period.
Below is our institution’s experience with managing patients on buprenorphine. Before January 2018, the practice at our institution was to withhold buprenorphine 72 hours before surgery, supplementing with opioid agonists to prevent withdrawal. However, this practice met multiple challenges: patients were fearful of stopping buprenorphine due to concerns of OUD relapse and opioid withdrawal; providers were concerned about the legal aspects of providing opioid agonists to patients with a history of OUD for reasons other than pain management; many buprenorphine prescribers objected to the practice of withholding buprenorphine without clear evidence of its benefit. Due to the lack of high-level evidence, we elected to convene a multidisciplinary clinician group to derive an integrated group assessment to guide clinical practice. Twenty clinicians experienced in providing perioperative care to patients with OUD attended our meeting from the following disciplines: perioperative pain management, chronic pain management, addiction medicine, psychology, psychiatry, and preoperative anesthesia evaluation. The group reviewed the literature on the analgesic efficacy of buprenorphine when combined with opioid agonists, buprenorphine receptor availability, and the perioperative and peripartum management of buprenorphine in patients with OUD.
The group reached a revised consensus for the perioperative management of patients on buprenorphine for OUD, implemented in January 2018 (described below).
This guideline aims to achieve the following interdependent goals:
To create a pain management strategy where buprenorphine can be continued perioperatively in patients with OUD, abolishing the need to prescribe opioid agonists before surgery for opioid withdrawal prevention.
To minimize the risk of nonprescribed or aberrant opioid use while not negatively impacting analgesia.
To facilitate a smooth transition back to the original buprenorphine maintenance regimen after surgical pain subsides. Maintaining patients on buprenorphine would avoid the uncomfortable withdrawal period that occurs before buprenorphine re-initiation, another potential time point where risk for relapse would be elevated.
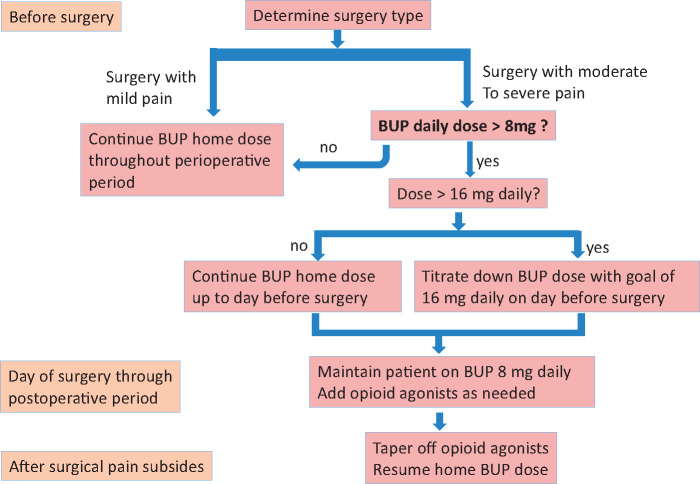
This guideline recommends a management algorithm based on the type of surgery and the dose of buprenorphine the patient is maintained on. For surgeries with minimal expected pain, we do not make changes to the patient’s buprenorphine maintenance regimen. For major surgeries where moderate to significant surgical pain is expected in patients who are on more than 16 mg of buprenorphine, we recommend tapering the buprenorphine dose to 16 mg of buprenorphine on the day before surgery. This dose effectively provides withdrawal suppression while allowing for adequate opioid receptor availability 24 hours later, as shown in previous studies [24,73]. From the day of surgery throughout the postoperative period, we recommend maintaining patients on an 8-mg daily dose—a conservative dose that allows additional opioid agonists to exert additive and synergistic analgesic effects. Once surgical pain subsides, patients are instructed to stop the use of postoperative opioid agonists and resume their baseline buprenorphine maintenance regimen (Figure 1).
Figure 1.
Algorithm for perioperative management of buprenorphine. BUP = buprenorphine.
There are currently no data to guide the optimal buprenorphine dose that should be used for analgesia to be effective when opioid agonists are administered concurrently. Recently, Lembke et al. [12] proposed an innovative approach in the perioperative management of buprenorphine. Similar to our approach, the authors recommended continuing buprenorphine at 12 mg or less for one to three days after surgery should other opioids be needed for breakthrough pain, after which buprenorphine can be returned to its preoperative dose, and supplemental opioids should be tapered as soon as possible. Although both approaches agree on continuing buprenorphine at a moderate dose throughout the perioperative period, there are some differences regarding timing and dose administration. Preoperatively, our protocol elected to reduce the daily buprenorphine dose to 16 mg, based on the fact that the blockade of euphoric effects from typical doses of abused opioids requires less than 20% mu morphine receptor availability, which is achieved with this dose [74]. We believe that this provides patients with OUD with more protection against potential relapse when their buprenorphine dose is reduced. The receptor binding time course study also showed that 24 hours after a 16-mg daily dose, the mu-opioid receptor availability recovers to approximately 40% [73]; thus a 16-mg dose of buprenorphine on the day before surgery is unlikely to adversely impact opioid analgesic effects on the day of surgery. We further reduced the dose to 8 mg daily starting on the day of surgery through the postoperative period to allow more mu-opioid receptor availability. It is unknown whether an 8-mg or 12-mg daily dose is more advantageous for postoperative pain control. Further studies are needed to establish the optimal dose of buprenorphine use in the perioperative period.
Regardless of the buprenorphine management approach used perioperatively, adjuvant analgesic techniques and opioid-sparing treatment modalities should be considered in all patients on chronic opioid maintenance therapy [78–81]. Additionally, nonopioid analgesics—including nonsteroidal anti-inflammatory drugs, gabapentinoids, alpha2 agonists, and NMDA receptor antagonists—should be utilized, depending on the surgical procedure and patient comorbidities, to enhance analgesic efficacy and limit opioid consumption [78,82]. Avoiding the psychological stress of perioperative pain as much as possible in all patients, particularly those with a history of OUD, is important, and each patient’s social and psychiatric issues should be addressed and optimally treated [78]. Management should begin early, at the time of preoperative assessment, and a collaborative multidisciplinary approach, incorporating pain management specialists, addiction medicine specialists, and psychiatrists, should be utilized when necessary. Patients should also be encouraged to be active participants in their treatment plans, and providers should address patient substance abuse history early to aid in elucidating optimal treatment plans. These discussions should include inquiry into each patient’s beliefs regarding pain, coping strategies effective during stressful situations, and fears and concerns regarding surgery and postoperative recovery. Patients should also be educated on what to expect following surgery, including the typical time course for acute pain and realistic goals for pain control. Ideally, these discussions should occur with individuals who have established connections with these patients and can follow them through the postoperative period.
Conclusions
Pain management in patients on long-term opioid therapy is inherently complex. In patients with a history of OUD, pain management is further complicated by the extensive neuropsychiatric and behavioral impairments endemic to addiction and opioid dependence. Although it has been accepted that treating acute pain in patients on buprenorphine is challenging due to its unique properties, there is evidence that the clinical implications of its pharmacology may not be as insurmountable as previously imagined and effective pain control is possible when patients are continued on buprenorphine perioperatively. Further investigations are needed to determine optimal buprenorphine dosing during the perioperative period. The studies highlighted in this review reveal that the dosing of buprenorphine and the time interval in which it is administered can either positively or negatively impact the efficacy of opioid analgesics used in combination. Broad guidelines recommending buprenorphine discontinuation before elective surgery, therefore, should be reconsidered as there is no high-level evidence that maintaining buprenorphine perioperatively leads to worsened outcomes. Furthermore, abrupt discontinuation of buprenorphine in the perioperative period while administering opioids with high misuse potential can trigger relapse. There are clinical instances where discontinuing buprenorphine is practical. For instance, in institutions where transmucosal buprenorphine is not available, it may not be feasible to continue buprenorphine maintenance therapy in the inpatient setting. In these instances, adjuvant analgesic techniques should be maximized for the treatment of postoperative pain, and members of the patient’s care team should collaborate to develop a suitable social, psychiatric, and pain management strategy to limit the risk of illicit opioid use. Similarly, there are instances where it is not feasible to make dose adjustments to buprenorphine before surgery and patients may be maintained on doses of buprenorphine greater than 16 mg perioperatively. Again, it is crucial to utilize adjuvant therapy and opioid-sparing techniques in these individuals as higher doses of opioid medications may be needed for pain control. Ultimately, further research is needed to determine the optimal perioperative management strategies for patients taking buprenorphine.
Acknowledgment
We would like to acknowledge the following experts who participated the multidisciplinary discussion and helped formulate our institutional perioperative buprenorphine management guideline at MGH: Drs. Gregory Acampora, Shihab Ahmed, Michael Bierer, Lucy Chen, Jeffrey Ecker, Mark Eisenberg, Brinda Kamdar, Ronald Kulich, Sara Lehrhoff, Jianren Mao, Laurie Shapiro, Nicole Zaneta Spence, Peter Stefanovich, Shane Volney, Sarah Wakeman, Jasmine Webb, Jenna Berube, and Christopher Shaw.
Funding sources: None.
Conflicts of interest: None.
Reference
- 1. Hedden SLK, Lipari R, Medley G, Tice P, Copello EAP, Kroutil LA. Behavioral health trends in the United States: Results from the 2014 National Survey on Drug Use and Health. 2015. Available at: https://www.samhsa.gov/data/sites/default/files/NSDUH-FRR1-2014/NSDUH-FRR1-2014.pdf (accessed October 23, 2018).
- 2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association Publishing; 2013. [Google Scholar]
- 3. CDC/NCHS. National Vital Statistics System, mortality. 2017. Available at: https://wonder.cdc.gov.
- 4. Florence CS, Zhou C, Luo F, Xu L. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Medical Care 2016;54(10):901–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: Systematic review and meta-analysis of cohort studies. BMJ 2017;357:j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weiss RD, Potter JS, Fiellin DA, et al. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: A 2-phase randomized controlled trial. Arch Gen Psychiatry 2011;68(12):1238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fudala PJ, Bridge TP, Herbert S, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. New Engl J Med 2003;349(10):949–58. [DOI] [PubMed] [Google Scholar]
- 8. Johnson RE, Fudala PJ, Payne R. Buprenorphine: Considerations for pain management. J Pain Symptom Manag 2005;29(3):297–326. [DOI] [PubMed] [Google Scholar]
- 9. Alford DP, Compton P, Samet JH. Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Ann Intern Med 2006;144(2):127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Substance Abuse and Mental Health Services Administration. Medication assisted treatment for opioid use disorders. 2016. Available at: https://www.federalregister.gov/documents/2016/07/08/2016-16120/medication-assisted-treatment-for-opioid-use-disorders (accessed October 23, 2018). [PubMed]
- 11. Anderson TA, Quaye ANA, Ward EN, et al. To stop or not, that is the question: Acute pain management for the patient on chronic buprenorphine. Anesthesiology 2017;126(6):1180–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lembke A, Ottestad E, Schmiesing C. Patients maintained on buprenorphine for opioid use disorder should continue buprenorphine through the perioperative period. Pain Med 2018;20(3):425–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clinical pharmacology of buprenorphine: Ceiling effects at high doses. Clin Pharmacol Ther 1994;55(5):569–80. [DOI] [PubMed] [Google Scholar]
- 14. Welsh C, Valadez-Meltzer A. Buprenorphine: A (relatively) new treatment for opioid dependence. Psychiatry (Edgmont) 2005;2(12):29–39. [PMC free article] [PubMed] [Google Scholar]
- 15. Orman JS, Keating GM. Buprenorphine/naloxone: A review of its use in the treatment of opioid dependence. Drugs 2009;69(5):577–607. [DOI] [PubMed] [Google Scholar]
- 16. Lalanne L, Ayranci G, Kieffer BL, Lutz PE. The kappa opioid receptor: From addiction to depression, and back. Front Psychiatry 2014;5:170.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li W, Sun H, Chen H, et al. Major depressive disorder and kappa opioid receptor antagonists. Transl Perioper Pain Med 2016;1(2):4–16. [PMC free article] [PubMed] [Google Scholar]
- 18. Elkader A, Sproule B. Buprenorphine: Clinical pharmacokinetics in the treatment of opioid dependence. Clin Pharmacokinet 2005;44(7):661–80. [DOI] [PubMed] [Google Scholar]
- 19. Substance Abuse and Mental Health Services Administration. SAMHSA/Treatment Improvement Protocol 63. Medications for Opioid Use Disorder: For Healthcare and Addiction Professionals, Policymakers, Patients, and Families. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2018. [PubMed] [Google Scholar]
- 20. Heit HA, Gourlay DL. Buprenorphine: New tricks with an old molecule for pain management. Clin J Pain 2008;24(2):93–7. [DOI] [PubMed] [Google Scholar]
- 21. Dahan A, Yassen A, Romberg R, et al. Buprenorphine induces ceiling in respiratory depression but not in analgesia. Br J Anaesth 2006;96(5):627–32. [DOI] [PubMed] [Google Scholar]
- 22. Mendelson J, Upton RA, Everhart ET, Jacob P 3rd, Jones RT. Bioavailability of sublingual buprenorphine. J Clin Pharmacol 1997;37(1):31–7. [DOI] [PubMed] [Google Scholar]
- 23. Christoph T, Kogel B, Schiene K, et al. Broad analgesic profile of buprenorphine in rodent models of acute and chronic pain. Eur J Pharmacol 2005;507(1–3):87–98. [DOI] [PubMed] [Google Scholar]
- 24. Greenwald MK, Johanson CE, Moody DE, et al. Effects of buprenorphine maintenance dose on mu-opioid receptor availability, plasma concentrations, and antagonist blockade in heroin-dependent volunteers. Neuropsychopharmacology 2003;28(11):2000–9. [DOI] [PubMed] [Google Scholar]
- 25. Zubieta J, Greenwald MK, Lombardi U, et al. Buprenorphine-induced changes in mu-opioid receptor availability in male heroin-dependent volunteers: A preliminary study. Neuropsychopharmacology 2000;23(3):326–34. [DOI] [PubMed] [Google Scholar]
- 26. Cicero TJ, Surratt HL, Inciardi J. Use and misuse of buprenorphine in the management of opioid addiction. J Opioid Manag 2007;3(6):302–8. [DOI] [PubMed] [Google Scholar]
- 27. Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev 2004;(3):CD002207. [DOI] [PubMed] [Google Scholar]
- 28.Indivior. Sublocade fact sheet. 2017. Available at: http://indivior.com/wp-content/uploads/2017/11/SUBLOCADE-Fact-Sheet.pdf (accessed October 23, 2018).
- 29.Buprenorphine (Probuphine & Sublocade): UnitedHealthcare commercial medical benefit drug policy. Probuphine - Braeburn Pharmaceuticals, Inc. Sublocade- Indivior Inc. 2018. Available at: https://www.uhcprovider.com/content/dam/provider/docs/public/policies/comm-medical-drug/probuphine-buprenorphine.pdf (accessed October 23, 2018).
- 30.Buprenex package insert. Reckitt Benckiser Pharmaceuticals Inc. 2007. Available at: https://www.naabt.org/documents/buprenex_PI.pdf (accessed October 23, 2018).
- 31. Plosker GL. Buprenorphine 5, 10 and 20 mug/h transdermal patch: A review of its use in the management of chronic non-malignant pain. Drugs 2011;71(18):2491–509. [DOI] [PubMed] [Google Scholar]
- 32.Purdue Pharma L.P. Butrans package insert. 2010. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021306s000lbl.pdf (accessed October 23, 2018).
- 33. Likar R. Transdermal buprenorphine in the management of persistent pain—safety aspects. Ther Clin Risk Manag 2006;2(1):115–25. [PMC free article] [PubMed] [Google Scholar]
- 34. Mercadante S, Ferrera P, Villari P. Is there a ceiling effect of transdermal buprenorphine? Preliminary data in cancer patients. Supportive Care Cancer 2007;15(4):441–4. [DOI] [PubMed] [Google Scholar]
- 35. Clement MP, Beuselinck B, Van Beek K, et al. The use of high dosages of transdermal buprenorphine for pain management in palliative cancer patients: A case study. Case Rep Oncol 2013;6(1):169–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pergolizzi JV Jr, Mercadante S, Echaburu AV, et al. The role of transdermal buprenorphine in the treatment of cancer pain: An expert panel consensus. Curr Med Res Opin 2009;25(6):1517–28. [DOI] [PubMed] [Google Scholar]
- 37. Mitra F, Chowdhury S, Shelley M, Williams G. A feasibility study of transdermal buprenorphine versus transdermal fentanyl in the long-term management of persistent non-cancer pain. Pain Med 2013;14(1):75–83. [DOI] [PubMed] [Google Scholar]
- 38.Endo Pharmaceuticals. Belbuca package insert. 2015. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207932s000lbl.pdf (accessed October 23, 2018).
- 39. Kögel B, Christoph T, Straβburger W, Friderichs E. Interaction of mu-opioid receptor agonists and antagonists with the analgesic effect of buprenorphine in mice. Eur J Pain 2005;9(5):599–611. [DOI] [PubMed] [Google Scholar]
- 40. Mercadante S, Villari P, Ferrera P, et al. Safety and effectiveness of intravenous morphine for episodic breakthrough pain in patients receiving transdermal buprenorphine. J Pain Symptom Manage 2006;32(2):175–9. [DOI] [PubMed] [Google Scholar]
- 41. Beltrutti D, Niv D. Pain relief after simultaneous administration of intravenous buprenorphine and intrathecal morphine in terminally ill patients; a report of two cases. Pain Clin 2000;12(2):121–3. [Google Scholar]
- 42. Beltrutti D, Niv D, Ben-Abraham R, Di Santo S, Weinbroum AA. Late antinociception and lower untoward effects of concomitant intrathecal morphine and intravenous buprenorphine in humans. J Clin Anesth 2002;14(6):441–6. [DOI] [PubMed] [Google Scholar]
- 43. Oifa S, Sydoruk T, White I, et al. Effects of intravenous patient-controlled analgesia with buprenorphine and morphine alone and in combination during the first 12 postoperative hours: A randomized, double-blind, four-arm trial in adults undergoing abdominal surgery. Clin Ther 2009;31(3):527–41. [DOI] [PubMed] [Google Scholar]
- 44. Hay JL, White JM, Bochner F, et al. Hyperalgesia in opioid-managed chronic pain and opioid-dependent patients. J Pain 2009;10(3):316–22. [DOI] [PubMed] [Google Scholar]
- 45. Compton P, Charuvastra VC, Ling W. Pain intolerance in opioid-maintained former opiate addicts: Effect of long-acting maintenance agent. Drug Alcohol Depend 2001;63(2):139–46. [DOI] [PubMed] [Google Scholar]
- 46. Athanasos P, Smith CS, White JM, et al. Methadone maintenance patients are cross-tolerant to the antinociceptive effects of very high plasma morphine concentrations. Pain 2006;120(3):267–75. [DOI] [PubMed] [Google Scholar]
- 47. Hansen LE, Stone GL, Matson CA, et al. Total joint arthroplasty in patients taking methadone or buprenorphine/naloxone preoperatively for prior heroin addiction: A prospective matched cohort study. J Arthroplasty 2016;31(8):1698–701. [DOI] [PubMed] [Google Scholar]
- 48. Vilkins AL, Bagley SM, Hahn KA, et al. Comparison of post-cesarean section opioid analgesic requirements in women with opioid use disorder treated with methadone or buprenorphine. J Addict Med 2017;11(5):397–401. [DOI] [PubMed] [Google Scholar]
- 49. Athanasos P, Ling W, Bochner F, White JM, Somogyi AA. Buprenorphine maintenance subjects are hyperalgesic and have no antinociceptive response to a very high morphine dose. Pain Med 2018;20(1):119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Huxtable CA, Roberts LJ, Somogyi AA, MacIntyre PE. Acute pain management in opioid-tolerant patients: A growing challenge. Anaesth Intensive Care 2011;39(5):804–23. [DOI] [PubMed] [Google Scholar]
- 51. Wachholtz A, Gonzalez G. Co-morbid pain and opioid addiction: Long term effect of opioid maintenance on acute pain. Drug Alcohol Depend 2014;145:143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Simpson GK, Jackson M. Perioperative management of opioid-tolerant patients. BJA Educ 2017;17(4):124–8. [Google Scholar]
- 53. Rapp SE, Ready LB, Nessly ML. Acute pain management in patients with prior opioid consumption: A case-controlled retrospective review. Pain 1995;61(2):195–201. [DOI] [PubMed] [Google Scholar]
- 54. Jonan AB, Kaye AD, Urman RD. Buprenorphine formulations: Clinical best practice strategies recommendations for perioperative management of patients undergoing surgical or interventional pain procedures. Pain Physician 2018;21(1):E1–12. [PubMed] [Google Scholar]
- 55. Bryson EO. The perioperative management of patients maintained on medications used to manage opioid addiction. Curr Opin Anaesthesiol 2014;27(3):359–64. [DOI] [PubMed] [Google Scholar]
- 56. McCormick Z, Chu SK, Chang-Chien GC, Joseph P. Acute pain control challenges with buprenorphine/naloxone therapy in a patient with compartment syndrome secondary to McArdle's disease: A case report and review. Pain Med 2013;14(8):1187–91. [DOI] [PubMed] [Google Scholar]
- 57. Huang A, Katznelson R, de Perrot M, Clarke H. Perioperative management of a patient undergoing Clagett window closure stabilized on Suboxone(R) for chronic pain: A case report. Can J Anaesth 2014;61(9):826–31. [DOI] [PubMed] [Google Scholar]
- 58. Gilmore T, Saccheti A, Cortese T. Buprenorphine/naloxone inhibition of remifentanil procedural sedation. Am J Emerg Med 2012;30(8):1655.e3–4. [DOI] [PubMed] [Google Scholar]
- 59. Brummett CM, Trivedi KA, Dubovoy AV, Berland DW. Dexmedetomidine as a novel therapeutic for postoperative pain in a patient treated with buprenorphine. J Opioid Manage 2009;5(3):175–9. [DOI] [PubMed] [Google Scholar]
- 60. Israel JS, Poore SO. The clinical conundrum of perioperative pain management in patients with opioid dependence: Lessons from two cases. Plast Reconstr Surg 2013;131(4):657e–8e. [DOI] [PubMed] [Google Scholar]
- 61. Chern SYS, Isserman R, Chen L, Ashburn M, Liu R. Perioperative pain management for patients on chronic buprenorphine: A case report. J Anesth Clin Res 2013;3(250):1000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Macintyre PE, Russell RA, Usher KA, Gaughwin M, Huxtable CA. Pain relief and opioid requirements in the first 24 hours after surgery in patients taking buprenorphine and methadone opioid substitution therapy. Anaesth Intensive Care 2013;41(2):222–30. [DOI] [PubMed] [Google Scholar]
- 63. Kornfeld H, Manfredi L. Effectiveness of full agonist opioids in patients stabilized on buprenorphine undergoing major surgery: A case series. Am J Ther 2010;17(5):523–8. [DOI] [PubMed] [Google Scholar]
- 64. Jones HE, Kaltenbach K, Heil SH, et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. New Engl J Med 2010;363(24):2320–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jones HE, O’Grady K, Dahne J, et al. Management of acute postpartum pain in patients maintained on methadone or buprenorphine during pregnancy. Am J Drug Alcohol Abuse 2009;35(3):151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jones HE, Johnson RE, Milio L. Post-cesarean pain management of patients maintained on methadone or buprenorphine. Am J Addict 2006;15(3):258–9. [DOI] [PubMed] [Google Scholar]
- 67. Meyer M, Paranya G, Keefer Norris A, Howard D. Intrapartum and postpartum analgesia for women maintained on buprenorphine during pregnancy. Eur J Pain 2010;14(9):939–43. [DOI] [PubMed] [Google Scholar]
- 68. Ling W, Hillhouse M, Domier C, et al. Buprenorphine tapering schedule and illicit opioid use. Addiction 2009;104(2):256–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bentzley BS, Barth KS, Back SE, Book SW. Discontinuation of buprenorphine maintenance therapy: Perspectives and outcomes. J Subst Abuse Treat 2015;52:48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sen S, Arulkumar S, Cornett EM, et al. New pain management options for the surgical patient on methadone and buprenorphine. Curr Pain Headache Rep 2016;20(3):16.. [DOI] [PubMed] [Google Scholar]
- 71. Sigmon SC, Dunn KE, Saulsgiver K, et al. A randomized, double-blind evaluation of buprenorphine taper duration in primary prescription opioid abusers. JAMA Psychiatry 2013;70(12):1347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Breen CL, Harris SJ, Lintzeris N, et al. Cessation of methadone maintenance treatment using buprenorphine: Transfer from methadone to buprenorphine and subsequent buprenorphine reductions. Drug Alcohol Depend 2003;71(1):49–55. [DOI] [PubMed] [Google Scholar]
- 73. Greenwald M, Johanson CE, Bueller J, et al. Buprenorphine duration of action: Mu-opioid receptor availability and pharmacokinetic and behavioral indices. Biol Psychiatry 2007;61(1):101–10. [DOI] [PubMed] [Google Scholar]
- 74. Greenwald MK, Comer SD, Fiellin DA. Buprenorphine maintenance and mu-opioid receptor availability in the treatment of opioid use disorder: Implications for clinical use and policy. Drug Alcohol Depend 2014;144:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Comer SD, Walker EA, Collins ED. Buprenorphine/naloxone reduces the reinforcing and subjective effects of heroin in heroin-dependent volunteers. Psychopharmacology 2005;181(4):664–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Malinoff HL, Barkin RL, Wilson G. Sublingual buprenorphine is effective in the treatment of chronic pain syndrome. Am J Ther 2005;12(5):379–84. [DOI] [PubMed] [Google Scholar]
- 77. Englberger W, Kogel B, Friderichs E, Strassburger W, Germann T. Reversibility of opioid receptor occupancy of buprenorphine in vivo. Eur J Pharmacol 2006;534(1–3):95–102. [DOI] [PubMed] [Google Scholar]
- 78. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: A clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists' Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain 2016;17(2):131–57. [DOI] [PubMed] [Google Scholar]
- 79. Block BM, Liu SS, Rowlingson AJ, et al. Efficacy of postoperative epidural analgesia: A meta-analysis. JAMA 2003;290(18):2455–63. [DOI] [PubMed] [Google Scholar]
- 80. Richman JM, Liu SS, Courpas G, et al. Does continuous peripheral nerve block provide superior pain control to opioids? A meta-analysis. Anesth Analg 2006;102(1):248–57. [DOI] [PubMed] [Google Scholar]
- 81. Wu CL, Cohen SR, Richman JM, et al. Efficacy of postoperative patient-controlled and continuous infusion epidural analgesia versus intravenous patient-controlled analgesia with opioids: A meta-analysis. Anesthesiology 2005;103(5):1079–88; quiz 109–10. [DOI] [PubMed] [Google Scholar]
- 82. Adams ML, Arminio GJ. Non-pharmacologic pain management intervention. Clin Podiatr Med Surg 2008;25(3):409–29; vi. [DOI] [PubMed] [Google Scholar]
- 83. Höflich AS, Langer M, Jagsch R, et al. Peripartum pain management in opioid dependent women. Eur J Pain 2012;16(4):574–84. [DOI] [PMC free article] [PubMed] [Google Scholar]