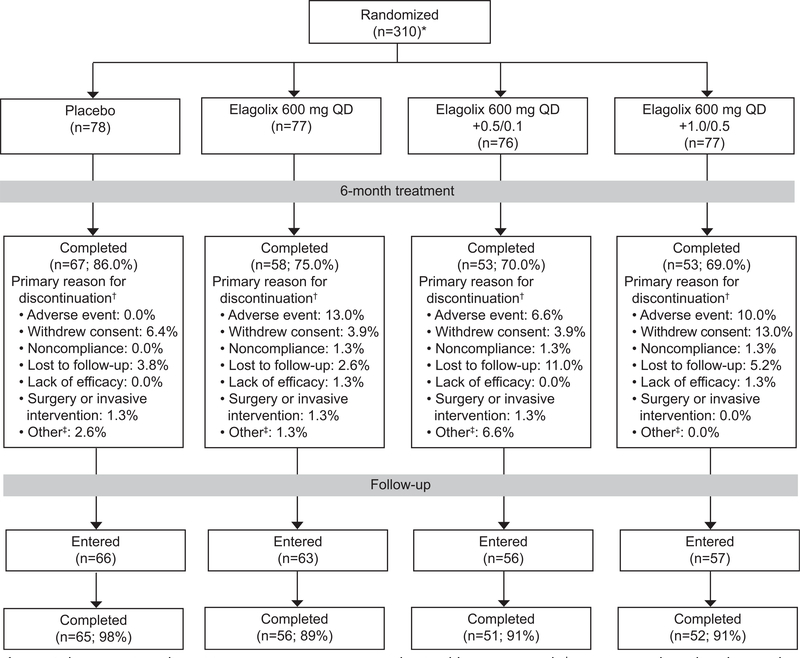
Fig. 3.
Cohort 2 patient disposition. *Two women were randomized but not treated. †Women may have listed more than one reason for premature discontinuation, but only the primary reasons are included. ‡Other category is a combination of pregnancy, exclusionary medication received, and other categories. QD, daily; 0.5/0.1, 0.5 mg estradiol/0.1 mg norethindrone acetate; 1.0/0.5, 1.0 mg estradiol/0.5 mg norethindrone acetate.