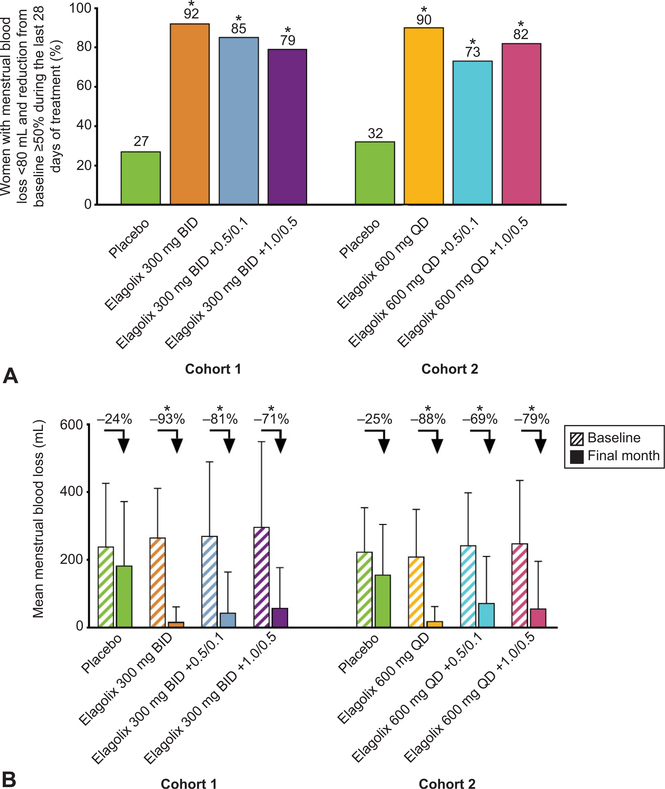
Fig. 4.
Percentage of women who met the composite primary end point (A) and mean menstrual blood loss at baseline and final month (B). Menstrual blood loss was measured from sanitary products by the alkaline hematin method. A. *Statistical significance vs placebo is indicated for P<.001. B. Arrows indicate the mean percent change from baseline to final month in menstrual blood loss. *Significance vs placebo is indicated for P<.001. BID, twice daily; QD, daily; 0.5/0.1, 0.5 mg estradiol/0.1 mg norethindrone acetate; 1.0/0.5, 1.0 mg estradiol/0.5 mg norethindrone acetate.