Fifty-eight patients with pathologically confirmed gliomas underwent preoperative 3D pseudocontinuous arterial spin-labeling and ROC curves were generated for parameters to distinguish high-grade from low-grade gliomas. Both maximum CBF and maximum relative CBF were significantly higher in high-grade than in low-grade gliomas. After adjustment for age, a higher maximum CBF and higher maximum relative CBF were associated with worse progression-free survival.
Abstract
BACKGROUND AND PURPOSE:
Previous studies showed conflicting results concerning the value of CBF maps obtained from arterial spin-labeling MR imaging in grading gliomas. This study was performed to investigate the effectiveness of CBF maps derived from 3D pseudocontinuous arterial spin-labeling in preoperatively assessing the grade, cellular proliferation, and prognosis of gliomas.
MATERIALS AND METHODS:
Fifty-eight patients with pathologically confirmed gliomas underwent preoperative 3D pseudocontinuous arterial spin-labeling. The receiver operating characteristic curves for parameters to distinguish high-grade gliomas from low-grade gliomas were generated. Pearson correlation analysis was used to assess the correlation among parameters. Survival analysis was conducted with Cox regression.
RESULTS:
Both maximum CBF and maximum relative CBF were significantly higher in high-grade gliomas than in low-grade gliomas (P < .001). The areas under the curve for maximum CBF and maximum relative CBF in distinguishing high-grade gliomas from low-grade gliomas were 0.828 and 0.863, respectively. Both maximum CBF and maximum relative CBF had no correlation with the Ki-67 index in all subjects and had a moderate negative correlation with the Ki-67 index in glioblastomas (r = −0.475, −0.534, respectively). After adjustment for age, a higher maximum CBF (P = .008) and higher maximum relative CBF (P = .005) were associated with worse progression-free survival in gliomas, while a higher maximum relative CBF (P = .033) was associated with better overall survival in glioblastomas.
CONCLUSIONS:
3D pseudocontinuous arterial spin-labeling–derived CBF maps are effective in preoperative evaluation of gliomas. Although gliomas with a higher blood flow are more malignant, glioblastomas with a lower blood flow are likely to be more aggressive.
Glioma is the most common intracranial malignant tumor, accounting for almost 80% of primary malignant brain tumors.1 Grading of gliomas is important for an optimal therapy plan and predicting outcome.2,3 According to the World Health Organization (WHO) criteria, gliomas can be classified into 4 groups: grades I–IV. Grade I and grade II gliomas are considered low-grade gliomas (LGGs), while grade III and grade IV gliomas are regarded as high-grade gliomas (HGGs).
Advanced MR imaging techniques, such as MR perfusion, have been shown to be more effective than conventional MR imaging techniques in grading gliomas.4,5 Dynamic susceptibility contrast perfusion imaging is the reference standard for evaluating tumor perfusion.6,7 However, this technique relies on the intravenous application of a contrast medium, which is not suitable for patients who are allergic to this medium or who have renal failure.8,9
Arterial spin-labeling (ASL) is a noninvasive MR perfusion imaging technique for obtaining CBF maps. Some previous studies based on pulsed ASL and continuous ASL (CASL) have shown that the ASL-derived CBF maps have potential value in grading gliomas8,10–15 and predicting their progression.9,16,17 However, although pseudocontinuous ASL (pCASL) is considered an improved method over pulsed ASL and CASL,18–20 a recent study reported that pCASL-derived CBF maps failed to accurately grade gliomas.21
On the other hand, according to many previous studies, gliomas with higher tumor blood flow are commonly more malignant.8–17 However, a recent study found a positive correlation between proliferation activity and levels of a hypoxia biomarker in glioblastoma (GBM),22 suggesting that GBM with a lower blood flow might be more aggressive. Hence, the correlation between relative CBF and the grade of malignancy might be more complex in gliomas.
The purpose of this study was to examine the value of the CBF maps derived from 3D pCASL in preoperatively assessing the grade, cellular proliferation, and prognosis of gliomas. Additionally, we performed a subgroup analysis on patients with GBM.
Materials and Methods
Patients
This was a retrospective study based on data collected from our prospective cohort of patients with gliomas who were hospitalized at the Department of Neurosurgery, Second Affiliated Hospital of Zhejiang University School of Medicine, between August 2013 and January 2015. This study was approved by the local ethics review board and was conducted in accordance with the ethical principles of the Declaration of Helsinki. Written informed consent was obtained from all participants. Fifty-eight patients with supratentorial cerebral gliomas, who underwent a preoperative MR imaging examination with a 3D pCASL sequence, were enrolled in this study. The preoperative Karnofsky Performance Scale (KPS) was administered in all patients when they were admitted to the hospital.
Imaging Data Acquisition
All subjects underwent MR imaging on a 3T system (Discovery MR750; GE Healthcare, Milwaukee, Wisconsin) with an 8-channel high-resolution receiver head coil. The CBF images were acquired with a 3D pCASL sequence with the following parameters: section thickness, 4 mm; number of sections, 36; FOV, 240 × 240 mm; matrix, 128 × 128; TR, 4632 ms; TE, 10.5 ms; flip angle, 111°; number of excitations, 3; labeling duration, 1450 ms; postlabeling duration, 1525 ms; and pixel bandwidth, 976.6 Hz/pixel. The scan time for this sequence was 4 minutes 29 seconds. In addition, a contrast-enhanced T2-FLAIR sequence was acquired after the injection of a gadolinium contrast agent.
Pathology
The median time interval between preoperative MR imaging and the operation was 4 days (range, 1–10 days). Three patients underwent only stereotactic biopsy, and the others underwent craniotomy. The histopathologic diagnosis was performed by pathologists on the basis of the WHO 2007 criteria.
The Ki-67 proliferating index was reported in 45 patients. In each case, areas with the highest number of positive-staining tumor nuclei were selected for calculating the Ki-67 index. According to previous literature,23,24 patients with a Ki-67 index of ≥30% were assigned to the high Ki-67 index group and patients with a Ki-67 index of <30% were assigned to the low Ki-67 index group.
Follow-Up
Three patients who underwent stereotactic biopsy and 2 patients who died of operative complications were excluded in the postoperative follow-up. Among the other 53 patients, only 2 (3.9%) were lost to follow-up. Thus, 51 patients were included in the survival analysis. The median follow-up time was 30 months (range, 24–36 months). Overall survival (OS) was defined as the time from diagnosis until either death or the time the patient was last known to be alive (censused), and progression-free survival (PFS) was defined as the time from diagnosis until tumor progression, recurrence, or death or when the patient was last known to be alive (censused).25
Image Processing and Analysis
The CBF images were all coregistered to the contrast-enhanced T2-FLAIR images by using SPM12 (www.fil.ion.ucl.ac.uk/spm). The analysis of the images was performed with ImageJ, Version 1.49 (National Institutes of Health, Bethesda, Maryland). The ROIs were manually placed on the contrast-enhanced T2-FLAIR images by 1 expert neuroradiologist with 20 years' experience, who was blinded to the pathology of the tumors. Before ROIs were drawn, the image section that was speculated to contain the tumor area with the highest tumor blood flow was chosen by referring to the CBF maps. Areas with an abnormal signal in the enhanced T2-FLAIR images were all included. Another rectangular ROI was drawn to include contralateral gray matter areas. Then ROIs were copied to the corresponding CBF maps, as shown in Fig 1. The maximum CBF values (CBFmax) in ROIs were obtained. Then the relative CBFmax (rCBFmax) was calculated by dividing the CBFmax in the tumor ROI by the CBFmax in the contralateral ROI.
Fig 1.
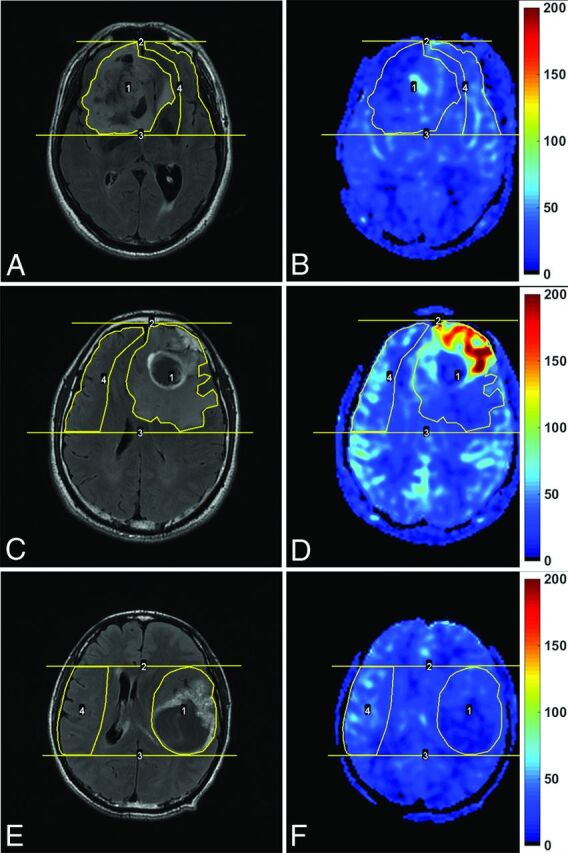
Enhanced T2-FLAIR images (A, C, and E) and CBF maps (B, D, and F) of a 69-year-old man with oligoastrocytoma (WHO grade II; Ki-67 index, 10%), a 42-year-old man with glioblastoma (WHO grade IV; Ki-67 index, 20%), and a 43-year-old man with glioblastoma (WHO grade IV; Ki-67 index, 60%), respectively. Note that blood flow is significantly elevated in the glioblastoma with a relatively low Ki-67 index, while it is not elevated in the glioblastoma with a very high Ki-67 index. The unit for CBF maps is milliliters/100 g/min.
Interobserver Concordance
Another reader, a junior neurosurgeon who was blinded to the pathology, also delineated the ROIs of all tumors. The measurements from this reader were only used for the assessment of interobserver concordance. The rCBFmax values measured by the 2 readers were compared by means of an intraclass correlation coefficient.
Statistical Analysis
All statistical analyses were performed with SPSS Statistics, Version 22 (IBM, Armonk, New York) and GraphPad Prism, Version 6.0 (GraphPad Software, San Diego, California). The significance level was set to α = .05. P < .05 was statistically significant.
The normality assumption was tested with the Kolmogorov-Smirnov test. The data were expressed as mean ± SD. Interobserver reliability was with the intraclass correlation coefficient based on a 2-way random-effects model. The Pearson correlation analysis was used to assess the correlation among parameters.
The 1-way ANOVA followed by the Fisher least significant difference test was used to compare differences in parameters among multiple groups. The differences in parameters between the HGG and the LGG groups were compared using the independent samples t test. The receiver operating characteristic (ROC) curves for parameters in distinguishing HGG from LGG were generated. Optimal cutoff values were derived from ROC curves, and sensitivity, specificity, predictive values, and accuracy were calculated on the basis of these best cutoff values.
Survival analysis was conducted with the Cox regression for both the univariate and multivariate analyses. Except for CBF parameters, 3 clinical features (age, sex, preoperative KPS) were also included in the survival analysis.
Results
Patient Characteristics
Among these 58 patients with gliomas, there were 27 women and 32 men, with a mean age of 49.5 years (range, 26–76 years) and a mean KPS score of 82.9 (range, 30–100). According to the WHO 2007 criteria, there were 13 patients diagnosed with WHO grade II (astrocytomas, n = 5; oligoastrocytomas, n = 4; oligodendrogliomas, n = 4), 17 patients diagnosed with WHO grade III (anaplastic astrocytomas, n = 6; anaplastic oligoastrocytomas, n = 7; anaplastic oligodendrogliomas, n = 4), and 28 patients diagnosed with WHO grade IV (GBM, n = 28).
Interobserver Concordance
The manifestations of an LGG, a GBM with a low Ki-67 index, and a GBM with a high Ki-67 index in contrast-enhanced T2-FLAIR images and CBF maps are shown in Fig 1. Our evaluation of the interobserver concordance for parameters showed excellent agreement. The intraclass correlation coefficients for the measurement of tumor CBFmax, contralateral CBFmax, and rCBFmax were as high as 0.995, 0.860, and 0.987, respectively. Furthermore, there was a very strong correlation between CBFmax and rCBFmax (r = 0.941).
Parameters in Each Type of Pathologic Tumor
The measured parameters in each type of pathologic tumor are summarized in Table 1. The CBFmax of GBMs was only significantly higher than that of astrocytomas (P = .002) and oligoastrocytomas (P = .005), while the rCBFmax of GBMs was significantly higher than that of astrocytomas (P < .001), oligoastrocytomas (P < .001), oligodendrogliomas (P = .032), anaplastic astrocytomas (P = .03), and anaplastic oligoastrocytomas (P = .027). Both the CBFmax and rCBFmax of GBMs were lower than those of anaplastic oligodendrogliomas without statistical significance (both P = .22).
Table 1:
Measurements of absolute CBFmax and rCBFmax in each type of pathologic tumor
| Grade/Histology | No. | CBFmax (mL/100 g/min) |
rCBFmax | |
|---|---|---|---|---|
| Tumor | Contralateral | |||
| Grade II (n = 13) | ||||
| Astrocytoma | 5 | 75.4 ± 10.2a,b | 83.2 ± 3.90 | 0.91 ± 0.18c,d |
| Oligoastrocytoma | 4 | 74.8 ± 12.1a,b | 93.2 ± 28.8 | 0.83 ± 0.16c,d |
| Oligodendroglioma | 4 | 109.8 ± 47.8e | 80.0 ± 9.6 | 1.38 ± 0.59e,f |
| Grade III (n = 17) | ||||
| Anaplastic astrocytoma | 6 | 127.2 ± 84.8e | 78.0 ± 23.6 | 1.51 ± 0.68e,f |
| Anaplastic oligoastrocytoma | 7 | 125.1 ± 71.9e | 82.4 ± 8.9 | 1.54 ± 0.88e,f |
| Anaplastic oligodendroglioma | 4 | 212.5 ± 87.5 | 78.8 ± 16.6 | 2.75 ± 1.02 |
| Grade IV (n = 28) | ||||
| Glioblastoma | 28 | 171.5 ± 58.7 | 77.0 ± 10.9 | 2.25 ± 0.79 |
P < .01, compared with glioblastoma.
P < .01, compared with anaplastic oligoastrocytoma.
P < .001, compared with glioblastoma.
P < .001, compared with anaplastic oligoastrocytoma.
P < .05, compared with anaplastic oligoastrocytoma.
P < .05, compared with glioblastoma.
Both the CBFmax and rCBFmax of anaplastic oligodendrogliomas were significantly higher than those of anaplastic astrocytomas (P = .035 and .013, respectively) and anaplastic oligoastrocytomas (P = .027 and .012, respectively), while those of oligodendrogliomas were higher than those of astrocytomas (P = .4 and .35, respectively) and oligoastrocytomas (P = .42 and .30, respectively) without statistical significances. Both the CBFmax and rCBFmax of anaplastic oligodendrogliomas were significantly higher than those of oligodendrogliomas (P = .021 and .012, respectively).
The mean CBFmax and rCBFmax were similar between astrocytomas and oligoastrocytomas, or between anaplastic astrocytomas and anaplastic oligoastrocytomas. No significant differences were detected among different pathologic types for contralateral CBFmax (P = .50).
Parameters in Each Tumor Grade
Both the absolute tumor CBFmax and rCBFmax increased with an increase in grade of glioma, as shown in Table 2. In all gliomas, both the CBFmax and rCBFmax of grade IV gliomas were significantly higher than those of grade II gliomas (P < .001), and those of grade III gliomas were significantly higher than those of grade II gliomas (P = .011 and .009, respectively), while those of grade IV gliomas were higher than those of grade III gliomas without statistical significance (P = .198 and .070, respectively). After oligodendrogliomas and anaplastic oligodendrogliomas were excluded, significant differences between grade III gliomas and grade IV gliomas were detected for both CBFmax and rCBFmax (P = .025 and .004, respectively).
Table 2:
Measurements of absolute CBFmax and rCBFmax in each WHO grade with or without the exclusion of oligodendrogliomas and anaplastic oligodendrogliomas
| Patients/Grades | No. | CBFmax (mL/100 g/min) |
rCBFmax | |
|---|---|---|---|---|
| Tumor | Contralateral | |||
| All | ||||
| Grade II | 13 | 85.8 ± 30.3 | 85.3 ± 16.4 | 1.03 ± 0.41 |
| Grade III | 17 | 146.4 ± 84.0b | 80.0 ± 16.1 | 1.81 ± 0.96c |
| Grade IV | 28 | 171.5 ± 58.7d | 77.0 ± 10.9 | 2.25 ± 0.15d |
| Excludeda | ||||
| Grade II | 9 | 75.1 ± 10.4 | 87.7 ± 18.6 | 0.88 ± 0.16 |
| Grade III | 13 | 126.1 ± 74.7e | 80.4 ± 16.6 | 1.52 ± 0.77b |
| Grade IV | 28 | 171.5 ± 58.7d,f | 77.0 ± 10.9 | 2.25 ± 0.15d,g |
With oligodendrogliomas and anaplastic oligodendrogliomas excluded. Note the following P values in each patient group:
P < .05, compared with grade II.
P < .01, compared with grade II.
P < .001, compared with grade II.
P = .05, compared with grade II.
P < .05, compared with grade III.
P < .01, compared with grade III.
Differentiation of LGG and HGG
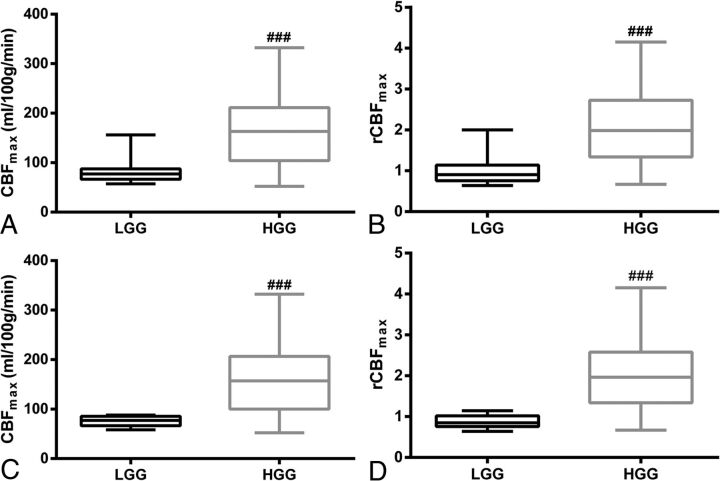
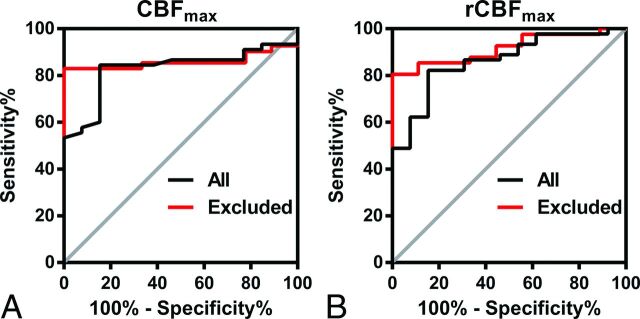
There were significant differences between LGG and HGG for both CBFmax and rCBFmax (P < .001), regardless of whether oligodendrogliomas and anaplastic oligodendrogliomas were excluded, as shown in Fig 2. The ROC curves for CBFmax and rCBFmax in distinguishing HGG from LGG are shown in Fig 3, and the results of the ROC analysis are shown in Table 3. The area under curve, best cutoff value, sensitivity, and specificity for CBFmax were 0.828, 91 (mL/100 g/min), 84.4%, and 84.6%, respectively, in all gliomas and were 0.859, 91 (mL/100 g/min), 82.9%, and 100%, respectively, after oligodendrogliomas and anaplastic oligodendrogliomas were excluded. Those for rCBFmax were 0.863, 1.19, 82.2%, and 84.6%, respectively, in all gliomas and were 0.916, 1.22, 80.5%, and 100%, respectively, after oligodendrogliomas and anaplastic oligodendrogliomas were excluded. Both CBFmax and rCBFmax values allowed HGGs to be distinguished from LGGs, and the differential diagnosis was improved after oligodendrogliomas and anaplastic oligodendrogliomas were excluded.
Fig 2.
Boxplots of CBFmax (A) and rCBFmax (B) in low-grade gliomas and high-grade gliomas for all subjects. The boxplots of CBFmax (C) and rCBFmax (D) in LGGs and HGGs after the oligodendrogliomas and anaplastic oligodendrogliomas were excluded. ### indicates P < .001, compared with LGG.
Fig 3.
Receiver operating characteristic curves of CBFmax (A) and rCBFmax (B) in distinguishing high- from low-grade gliomas, without (black line) or with (red line) oligodendrogliomas and anaplastic oligodendrogliomas excluded.
Table 3:
ROC curve analyses of CBFmax and rCBFmax in discriminating high- and low-grade gliomas
| Parameters/Patients | AUC | Youden Index | Cutoff Value | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|---|
| CBFmax | ||||||||
| All | 0.828 | 0.690 | 91 mL/100 g/min | 84.4 | 84.6 | 95.0 | 61.1 | 84.5 |
| Excludeda | 0.859 | 0.829 | 91 mL/100 g/min | 82.9 | 100 | 100 | 56.3 | 86.0 |
| rCBFmax | ||||||||
| All | 0.863 | 0.668 | 1.19 | 82.2 | 84.6 | 94.9 | 57.9 | 82.8 |
| Excludeda | 0.916 | 0.805 | 1.22 | 80.5 | 100 | 100 | 52.9 | 84.0 |
Note:—AUC indicates area under the curve; PPV, positive predictive value; NPV, negative predictive value.
With oligodendrogliomas and anaplastic oligodendrogliomas excluded.
Correlation with the Ki-67 Index
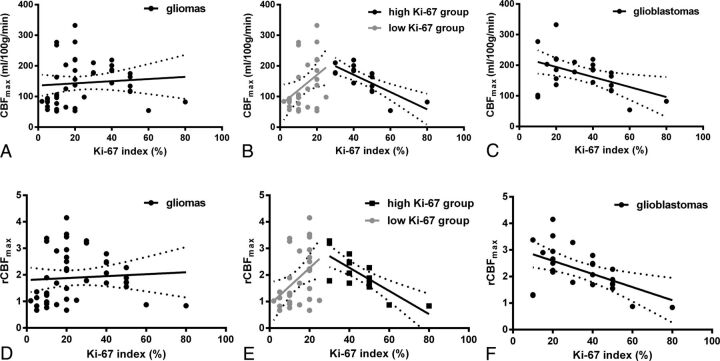
In all patients, as shown in Fig 4, both CBFmax (P = .565) and rCBFmax (P = .652) values were not correlated with the Ki-67 index. However, there was a moderate positive association between CBFmax and the Ki-67 index in the low Ki-67 index group (P = .029, r = 0.399), and a high inverse association between CBFmax and the Ki-67 index in the high Ki-67 index group (P < .001, r = −0.775). Similarly, rCBFmax had a moderate positive correlation with the Ki-67 index in the lower Ki-67 index group (P = .017, r = 0.432) and a high inverse correlation with the Ki-67 index in the high Ki-67 index group (P < .001, r = −0.784). In addition, in GBM, both CBFmax (P = .017, r = −0.475) and rCBFmax (P = .006, r = −0.534) showed a moderate negative correlation with the Ki-67 index.
Fig 4.
The linear regression of CBFmax (A–C) and rCBFmax (D–F) with the Ki-67 index in all subjects (A and D), in the low and high Ki-67 groups (B and E), and in glioblastomas (C and F). The low Ki-67 group included patients with a Ki-67 index of <30%, and the high Ki-67 group included patients with a Ki-67 index of ≥30%.
Survival Analysis
Fifty-one patients (13 grade II, 13 grade III, 25 grade IV) were included in the survival analysis. Table 4 shows the results obtained with the univariate Cox model for PFS and OS in both gliomas and GBMs. Sex had no association with PFS or OS in both gliomas and GBMs (all data, P > .5). In gliomas, higher CBFmax, higher rCBFmax, older age, and lower KPS were associated with worse PFS (hazard ratio [HR] = 1.005, 1.670, 1.036, 0.971, respectively; all data, P < .05), while CBFmax and rCBFmax were not prognosis factors for OS (P = .244 and .232, respectively). In GBMs, lower CBFmax, lower rCBFmax, and older age tended to be associated with worse OS without statistical significances (HR = 0.993, 0.563, 1.030, respectively; P = .070, .065, and .103, respectively), while CBFmax and rCBFmax were not associated with PFS (P = .497 and .644, respectively).
Table 4:
Univariate Cox model for progression-free survival and overall survival in patients with gliomas and glioblastomas
| Variables | PFS |
OS |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Gliomas (n = 51) | ||||||
| Age | 1.036 | 1.009–1.063 | .008 | 1.043 | 1.014–1.073 | .004 |
| Sexa | 0.957 | 0.670–1.368 | .811 | 0.896 | 0.606–1.325 | .582 |
| KPS | 0.971 | 0.953–0.989 | .002 | 0.970 | 0.952–0.988 | .002 |
| CBFmax | 1.005 | 1.001–1.010 | .027 | 1.003 | 0.998–1.008 | .244 |
| rCBFmax | 1.670 | 1.149–2.427 | .007 | 1.246 | 0.869–1.785 | .232 |
| GBM (n = 25) | ||||||
| Age | 1.036 | 1.002–1.072 | .039 | 1.030 | 0.994–1.067 | .103 |
| Sexa | 0.851 | 0.547–1.323 | .851 | 0.870 | 0.548–1.381 | .555 |
| KPS | 0.986 | 0.963–1.008 | .215 | 0.987 | 0.965–1.009 | .242 |
| CBFmax | 0.997 | 0.989–1.005 | .497 | 0.993 | 0.985–1.001 | .070 |
| rCBFmax | 0.865 | 0.467–1.601 | .644 | 0.563 | 0.306–1.035 | .065 |
Female versus male.
Either CBFmax or rCBFmax were included in the multivariate Cox analysis for PFS in gliomas, together with age and KPS. Furthermore, CBFmax or rCBFmax were also included in the multivariate Cox analysis for OS in GBM, together with age. The results showed that rCBFmax was a significant independent prognostic factor for PFS in gliomas (P = .005) and OS in GBMs (P = .033), while CBFmax was only a significant independent prognosis factor for PFS in gliomas (P = .008), shown in Table 5. After adjustment for age, higher CBFmax (HR = 1.007) and higher rCBFmax (HR = 1.707) were associated with worse PFS in gliomas, while higher rCBFmax (HR = 0.490) was associated with better OS in GBM.
Table 5:
Multivariate Cox model for progression-free survival in patients with gliomas and overall survival in patients with glioblastomas
| Parameters | Variables | PFS in Gliomas |
OS in GBM |
||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | ||
| Multivariate Cox model including CBFmax | CBFmax | 1.007 | 1.002–1.012 | .008 | 0.992 | 0.984–1.001 | .066 |
| Age | 1.043 | 1.015–1.071 | .002 | 1.030 | 0.995–1.067 | .097 | |
| KPSa | – | – | .131b | – | – | – | |
| Multivariate Cox model including rCBFmax | rCBFmax | 1.707 | 1.174–2.483 | .005 | 0.490 | 0.254–0.943 | .033 |
| Age | 1.038 | 1.011–1.065 | .006 | 1.037 | 1.001–1.074 | .045 | |
| KPSa | – | – | .233b | – | – | – | |
KPS was not included in the multivariate Cox analysis for OS in glioblastomas.
Only the P value was presented for the variable, which was excluded from the Cox model with P > .10.
Discussion
In this study, 3D pCASL-derived CBF maps were found to be effective in preoperatively assessing the grade and prognosis in gliomas. Another interesting finding was that CBFmax and rCBFmax showed a dual relationship with the degree of malignancy in gliomas. In accordance with previous studies based on pulsed ASL and CASL,8–17 both CBFmax and rCBFmax obtained from pCASL increased with increasing grade of gliomas, and gliomas with higher CBFmax and rCBFmax were associated with worse PFS. These findings suggest that gliomas with higher rCBFmax are associated with a higher degree of malignancy. However, in GBMs, both CBFmax and rCBFmax were found to have a significant negative correlation with the Ki-67 index. Also, GBMs with a lower rCBFmax were associated with worse OS after adjustment for age. Thus, GBMs with lower blood flow seemed to be more aggressive.
Unlike quantification methods of DSC MR imaging, which require an accurate arterial input function, 1 advantage of the ASL sequence is that it can provide an absolute quantification of CBF.9 In our study, a very strong correlation between CBFmax and rCBFmax was reported. The results of rCBFmax were similar to those of CBFmax in grading gliomas and evaluating the Ki-67 index, while rCBFmax seemed to be more valuable in predicting the prognosis of gliomas and GBMs. These results suggest that ASL-derived absolute CBF is also useful in evaluating gliomas.
However, compared with the DSC perfusion MR imaging, one limitation of the application of ASL is the relatively low SNR.15,20 CASL is an improved method, providing a higher tagging efficiency compared with pulsed ASL.18 However, because of magnetization transfer effects, the magnetization in the target tissues will be reduced, which limits the application of this method.18 In 2005, Garcia el al19 proposed the pCASL method to overcome this disadvantage, and this method can improve not only the SNR but also the accuracy of CBF measurements.18,20 Previous studies have shown that the pulsed ASL–derived and CASL-derived CBF maps have potential value in grading gliomas.8,10–15 In the current study, pCASL-derived CBF maps were also found to be effective in grading gliomas. However, a previous study indicated that pCASL-derived CBF maps were unable to grade gliomas, in contradiction to our results.21 In that study, compared with relative CBF derived from the dynamic contrast-enhanced MR imaging, the ASL-derived relative CBF was found to be relatively higher in LGGs but lower in HGGs. The potential explanation is not clear. More research may be required to evaluate the reliability of the CBF maps derived from pCASL.
Oligodendrogliomas and anaplastic oligodendrogliomas exhibit elevated relative CBV compared with astrocytic tumors of the same histologic grade.26,27 Similarly, our study also found that both CBFmax and rCBFmax were clearly elevated in oligodendrogliomas (not statistically significant) and anaplastic oligodendrogliomas (statistically significant) compared with other pathologic subtypes of the same histologic grade. In accordance with previous studies based on relative CBV,26 ability of the CBFmax and rCBFmax values in differentiating HGGs from LGGs improved after we excluded oligodendrogliomas and anaplastic oligodendrogliomas.
Furthermore, our results demonstrated a significantly higher CBFmax and rCBFmax in anaplastic oligodendrogliomas than in oligodendrogliomas. These results conflicted with those of a previous study based on CASL-derived CBFmax.12 However, a recent study by Fellah et al,28 based on DSC-derived relative CBF, reported a result similar to that in our study. Several previous studies have also shown a significantly higher relative CBV in anaplastic oligodendrogliomas than in oligodendrogliomas,28–31 while some other studies have reported a conflicting result.32–34 The ability of perfusion parameters to distinguish oligodendrogliomas and anaplastic oligodendrogliomas needs to be evaluated further.
A previous study by Mayer et al22 showed a positive correlation between proliferative activity and the level of a hypoxia biomarker in GBM. This molecular pathology finding is consistent with our finding that the Ki-67 index had a negative correlation with CBFmax and rCBFmax in GBMs. However, the potential mechanism is not clear. Evans et al35 found that higher grade gliomas were associated with more hypoxia. It is widely accepted that hypoxia will lead to the activation of the transcription of hypoxia-inducible factor-1 via stabilization of its α subunit.36–38 One potential mechanism of this may result in lower tumor blood flow in GBMs, which causes severe hypoxia and, in turn, the activation of the transcription of hypoxia-inducible factor-1, leading to high proliferative activity through further downstream pathways. Another potential mechanism might be due to the inability of angiogenesis to keep up with tumor cell proliferation in certain GBMs that have a high proliferative activity. The explicit mechanism needs to be investigated further.
On the other hand, our study also found that GBMs with a lower rCBFmax were associated with a worse OS but showed no association with PFS. This finding also suggests that a GBM with lower blood flow may cause severe hypoxia, causing it to become increasingly malignant. Some other studies have also found that hypoxia in GBM is related to a poor prognosis,39 yet 2 previous studies based on ASL-derived CBF maps have reported a contradictory result.16,17 However, 1 of those contradictory studies involved only 18 gliomas,16 and another contradictory study had only performed qualitative analysis,17 which reduced their reliability. It is well-known that the activity of hypoxia-inducible factor-1α–mediated pathways due to hypoxia will lead to migration and invasion of tumor cells.40,41 In addition, hypoxia is known to induce resistance to radiation therapy and chemotherapy through several mechanisms.42,43 This could explain why GBMs with a lower blood flow were more aggressive and were associated with a worse OS.
However, a previous study by Law et al44 in 2008 found that GBMs with high relative CBV were significantly associated with a poor OS, while another study by Deike et al45 in 2016 suggested that GBMs with a low blood supply may be associated with poor OS. The difference in results between these studies may be due to the different postoperative treatment strategies. Currently, concomitant radiochemotherapy has become the standard treatment for GBMs, and sometimes bevacizumab is also used to treat recurrent GBMs. Thus, resistance to radiation therapy and chemotherapy is now playing a more important role than before in the treatment of GBMs and will clearly influence the OS of patients with GBMs. A prospective study enrolling relatively large samples is needed to accurately evaluate the role of perfusion parameters in predicting the outcome of GBMs.
There were some limitations in our research. First, the sample size used for this study was not very large; therefore, some of the results may not be completely reliable, especially the results from the subgroup analysis. Further studies enrolling larger samples are needed to verify these results. Second, the postcontrast T2-FLAIR images were consulted when drawing the ROIs, so the ROI selection method presented in our study was not an accurate portrayal of a blind study. However, the resulting bias is hardly avoided, and it has also existed in many previous studies.8–14 Compared with previous ROI selection methods, the method presented in our study is relatively objective, with an excellent intraclass correlation coefficient. Third, molecular pathology for gliomas, such as 1p19q deletion and IDH1/2 mutations, was not routinely examined in our study. Further studies dividing gliomas or GBMs into subgroups by molecular pathology may provide further useful information.
Conclusions
3D pCASL-derived CBF maps are effective in preoperative evaluation of gliomas. Although gliomas with higher blood flow are associated with a higher degree of malignancy, GBMs with a lower blood flow are likely to be more aggressive.
Acknowledgments
We thank Professor Yi Shen from the Department of Public Health, Zhejiang University School of Medicine, for his assistance in statistics, and Harry Lee from Zhejiang University for his assistance in editing the manuscript.
ABBREVIATIONS:
- ASL
arterial spin-labeling
- CASL
continuous ASL
- GBM
glioblastoma
- HGG
high-grade glioma
- HR
hazard ratio
- KPS
Karnofsky Performance Scale
- LGG
low-grade glioma
- max
maximum
- OS
overall survival
- pCASL
pseudocontinuous ASL
- PFS
progression-free survival
- ROC
receiver operating characteristic
- WHO
World Health Organization
Footnotes
Disclosures: Qiang Zeng—RELATED: Grant: Hangzhou Municipal Health Bureau; UNRELATED: Employment: Second Affiliated Hospital of Zhejiang University College of Medicine. Biao Jiang—RELATED: Grant: Zhejiang Provincial Natural Science Foundation Committee; UNRELATED: Employment: Second Affiliated Hospital of Zhejiang University College of Medicine. Chenhan Ling—RELATED: Grant: Hangzhou Municipal Health Bureau; UNRELATED: Employment: Second Affiliated Hospital of Zhejiang University College of Medicine. Fei Dong—UNRELATED: Employment: Second Affiliated Hospital of Zhejiang University College of Medicine. Jianmin Zhang—UNRELATED: Employment: Second Affiliated Hospital of Zhejiang University College of Medicine; Grants/Grants Pending: The National Natural Science Foundation of China.
This work was supported by the Natural Science Foundation of Zhejiang (grant No. LY13H180006) and the Medicine and Health Research Foundation of Hangzhou (grant No. 20140633B25).
References
- 1. Schwartzbaum JA, Fisher JL, Aldape KD, et al. Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol 2006;2:494–503 10.1038/ncpneuro0289 [DOI] [PubMed] [Google Scholar]
- 2. Weller M, van den Bent M, Hopkins K, et al. ; European Association for Neuro-Oncology (EANO) Task Force on Malignant Glioma. EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol 2014;15:e395–e403 10.1016/S1470-2045(14)70011-7 [DOI] [PubMed] [Google Scholar]
- 3. Soffietti R, Baumert BG, Bello L, et al. ; European Federation of Neurological Societies. Guidelines on the management of low-grade gliomas: report of an EFNS-EANO Task Force. Eur J Neurol 2010;17:1124–33 10.1111/j.1468-1331.2010.03151.x [DOI] [PubMed] [Google Scholar]
- 4. Roy B, Gupta RK, Maudsley AA, et al. Utility of multiparametric 3-T MRI for glioma characterization. Neuroradiology 2013;55:603–13 10.1007/s00234-013-1145-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nguyen TB, Cron GO, Perdrizet K, et al. Comparison of the diagnostic accuracy of DSC- and dynamic contrast-enhanced MRI in the preoperative grading of astrocytomas. AJNR Am J Neuroradiol 2015;36:2017–22 10.3174/ajnr.A4398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Knopp EA, Cha S, Johnson G, et al. Glial neoplasms: dynamic contrast-enhanced T2*-weighted MR imaging. Radiology 1999;211:791–98 10.1148/radiology.211.3.r99jn46791 [DOI] [PubMed] [Google Scholar]
- 7. Law M, Yang S, Babb JS, et al. Comparison of cerebral blood volume and vascular permeability from dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. AJNR Am J Neuroradiol 2004;25:746–55 [PMC free article] [PubMed] [Google Scholar]
- 8. Furtner J, Schöpf V, Schewzow K, et al. Arterial spin-labeling assessment of normalized vascular intratumoral signal intensity as a predictor of histologic grade of astrocytic neoplasms. AJNR Am J Neuroradiol 2014;35:482–89 10.3174/ajnr.A3705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rau MK, Braun C, Skardelly M, et al. Prognostic value of blood flow estimated by arterial spin labeling and dynamic susceptibility contrast-enhanced MR imaging in high-grade gliomas. J Neurooncol 2014;120:557–66 10.1007/s11060-014-1586-z [DOI] [PubMed] [Google Scholar]
- 10. Fudaba H, Shimomura T, Abe T, et al. Comparison of multiple parameters obtained on 3T pulsed arterial spin-labeling, diffusion tensor imaging, and MRS and the Ki-67 labeling index in evaluating glioma grading. AJNR Am J Neuroradiol 2014;35:2091–98 10.3174/ajnr.A4018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim HS, Kim SY. A prospective study on the added value of pulsed arterial spin-labeling and apparent diffusion coefficients in the grading of gliomas. AJNR Am J Neuroradiol 2007;28:1693–99 10.3174/ajnr.A0674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chawla S, Wang S, Wolf RL, et al. Arterial spin-labeling and MR spectroscopy in the differentiation of gliomas. AJNR Am J Neuroradiol 2007;28:1683–89 10.3174/ajnr.A0673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Warmuth C, Gunther M, Zimmer C. Quantification of blood flow in brain tumors: comparison of arterial spin labeling and dynamic susceptibility-weighted contrast-enhanced MR imaging. Radiology 2003;228:523–32 10.1148/radiol.2282020409 [DOI] [PubMed] [Google Scholar]
- 14. Kim MJ, Kim HS, Kim JH, et al. Diagnostic accuracy and interobserver variability of pulsed arterial spin labeling for glioma grading. Acta Radiol 2008;49:450–57 10.1080/02841850701881820 [DOI] [PubMed] [Google Scholar]
- 15. Wolf RL, Wang J, Wang S, et al. Grading of CNS neoplasms using continuous arterial spin labeled perfusion MR imaging at 3 Tesla. J Magn Reson Imaging 2005;22:475–82 10.1002/jmri.20415 [DOI] [PubMed] [Google Scholar]
- 16. Furtner J, Bender B, Braun C, et al. Prognostic value of blood flow measurements using arterial spin labeling in gliomas. PLoS One 2014;9:e99616 10.1371/journal.pone.0099616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qiao XJ, Ellingson BM, Kim HJ, et al. Arterial spin-labeling perfusion MRI stratifies progression-free survival and correlates with epidermal growth factor receptor status in glioblastoma. AJNR Am J Neuroradiol 2015;36:672–77 10.3174/ajnr.A4196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wong EC. New developments in arterial spin labeling pulse sequences. NMR Biomed 2013;26:887–91 10.1002/nbm.2954 [DOI] [PubMed] [Google Scholar]
- 19. Garcia DM, De Bazelaire C, Alsop D. Pseudo-continuous flow driven adiabatic inversion for arterial spin labeling. Proc Int Soc Magn Reson Med 2005;13:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fernández-Seara MA, Edlow BL, Hoang A, et al. Minimizing acquisition time of arterial spin labeling at 3T. Magn Reson Med 2008;59:1467–71 10.1002/mrm.21633 [DOI] [PubMed] [Google Scholar]
- 21. Roy B, Awasthi R, Bindal A, et al. Comparative evaluation of 3-dimensional pseudocontinuous arterial spin labeling with dynamic contrast-enhanced perfusion magnetic resonance imaging in grading of human glioma. J Comput Assist Tomogr 2013;37:321–26 10.1097/RCT.0b013e318282d7e2 [DOI] [PubMed] [Google Scholar]
- 22. Mayer A, Schneider F, Vaupel P, et al. Differential expression of HIF-1 in glioblastoma multiforme and anaplastic astrocytoma. Int J Oncol 2012;41:1260–70 10.3892/ijo.2012.1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schaffel R, Hedvat CV, Teruya-Feldstein J, et al. Prognostic impact of proliferative index determined by quantitative image analysis and the International Prognostic Index in patients with mantle cell lymphoma. Ann Oncol 2010;21:133–39 10.1093/annonc/mdp495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bottini A, Berruti A, Brizzi MP, et al. Cytotoxic and antiproliferative activity of the single agent epirubicin versus epirubicin plus tamoxifen as primary chemotherapy in human breast cancer: a single-institution phase III trial. Endocr Relat Cancer 2005;12:383–92 10.1677/erc.1.00945 [DOI] [PubMed] [Google Scholar]
- 25. Jain R, Poisson LM, Gutman D, et al. Outcome prediction in patients with glioblastoma by using imaging, clinical, and genomic biomarkers: focus on the nonenhancing component of the tumor. Radiology 2014;272:484–93 10.1148/radiol.14131691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lev MH, Ozsunar Y, Henson JW, et al. Glial tumor grading and outcome prediction using dynamic spin-echo MR susceptibility mapping compared with conventional contrast-enhanced MR: confounding effect of elevated rCBV of oligodendroglimoas [corrected]. AJNR Am J Neuroradiol 2004;25:214–21 [PMC free article] [PubMed] [Google Scholar]
- 27. Cha S, Tihan T, Crawford F, et al. Differentiation of low-grade oligodendrogliomas from low-grade astrocytomas by using quantitative blood-volume measurements derived from dynamic susceptibility contrast-enhanced MR imaging. AJNR Am J Neuroradiol 2005;26:266–73 [PMC free article] [PubMed] [Google Scholar]
- 28. Fellah S, Caudal D, De Paula AM, et al. Multimodal MR imaging (diffusion, perfusion, and spectroscopy): is it possible to distinguish oligodendroglial tumor grade and 1p/19q codeletion in the pretherapeutic diagnosis? AJNR Am J Neuroradiol 2013;34:1326–33 10.3174/ajnr.A3352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kapoor GS, Gocke TA, Chawla S, et al. Magnetic resonance perfusion-weighted imaging defines angiogenic subtypes of oligodendroglioma according to 1p19q and EGFR status. J Neurooncol 2009;92:373–86 10.1007/s11060-009-9880-x [DOI] [PubMed] [Google Scholar]
- 30. Whitmore RG, Krejza J, Kapoor GS, et al. Prediction of oligodendroglial tumor subtype and grade using perfusion weighted magnetic resonance imaging. J Neurosurg 2007;107:600–09 10.3171/JNS-07/09/0600 [DOI] [PubMed] [Google Scholar]
- 31. Spampinato MV, Smith JK, Kwock L, et al. Cerebral blood volume measurements and proton MR spectroscopy in grading of oligodendroglial tumors. AJR Am J Roentgenol 2007;188:204–12 10.2214/AJR.05.1177 [DOI] [PubMed] [Google Scholar]
- 32. Jenkinson MD, Smith TS, Joyce KA, et al. Cerebral blood volume, genotype and chemosensitivity in oligodendroglial tumours. Neuroradiology 2006;48:703–13 10.1007/s00234-006-0122-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hilario A, Ramos A, Perez-Nuñez A, et al. The added value of apparent diffusion coefficient to cerebral blood volume in the preoperative grading of diffuse gliomas. AJNR Am J Neuroradiol 2012;33:701–07 10.3174/ajnr.A2846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xu M, See SJ, Ng WH, et al. Comparison of magnetic resonance spectroscopy and perfusion-weighted imaging in presurgical grading of oligodendroglial tumors. Neurosurgery 2005;56:919–26 10.1227/01.NEU.0000157957.67708.3E [DOI] [PubMed] [Google Scholar]
- 35. Evans SM, Judy KD, Dunphy I, et al. Hypoxia is important in the biology and aggression of human glial brain tumors. Clin Cancer Res 2004;10:8177–84 10.1158/1078-0432.CCR-04-1081 [DOI] [PubMed] [Google Scholar]
- 36. Korkolopoulou P, Patsouris E, Konstantinidou AE, et al. Hypoxia-inducible factor 1alpha/vascular endothelial growth factor axis in astrocytomas: associations with microvessel morphometry, proliferation and prognosis. Neuropathol Appl Neurobiol 2004;30:267–78 10.1111/j.1365-2990.2003.00535.x [DOI] [PubMed] [Google Scholar]
- 37. Mashiko R, Takano S, Ishikawa E, et al. Hypoxia-inducible factor 1alpha expression is a prognostic biomarker in patients with astrocytic tumors associated with necrosis on MR image. J Neurooncol 2011;102:43–50 10.1007/s11060-010-0292-8 [DOI] [PubMed] [Google Scholar]
- 38. Birner P, Piribauer M, Fischer I, et al. Vascular patterns in glioblastoma influence clinical outcome and associate with variable expression of angiogenic proteins: evidence for distinct angiogenic subtypes. Brain Pathol 2003;13:133–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bekaert L, Valable S, Lechapt-Zalcman E, et al. [18F]-FMISO PET study of hypoxia in gliomas before surgery: correlation with molecular markers of hypoxia and angiogenesis. Eur J Nucl Med Mol Imaging 2017;44:1383–92 10.1007/s00259-017-3677-5 [DOI] [PubMed] [Google Scholar]
- 40. Fujiwara S, Nakagawa K, Harada H, et al. Silencing hypoxia-inducible factor-1alpha inhibits cell migration and invasion under hypoxic environment in malignant gliomas. Int J Oncol 2007;30:793–802 [PubMed] [Google Scholar]
- 41. Zagzag D, Lukyanov Y, Lan L, et al. Hypoxia-inducible factor 1 and VEGF upregulate CXCR4 in glioblastoma: implications for angiogenesis and glioma cell invasion. Lab Invest 2006;86:1221–32 10.1038/labinvest.3700482 [DOI] [PubMed] [Google Scholar]
- 42. Toth RK, Warfel NA. Strange bedfellows: nuclear factor, erythroid 2-like 2 (Nrf2) and hypoxia-inducible factor 1 (HIF-1) in tumor hypoxia. Antioxidants 2017;6:27 10.3390/antiox6020027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu H, Cai Y, Zhang Y, et al. Development of a hypoxic radiosensitizer-prodrug liposome delivery DNA repair inhibitor Dbait combination with radiotherapy for glioma therapy. Adv Healthc Mater 2017;6:1601377 10.1002/adhm.201601377 [DOI] [PubMed] [Google Scholar]
- 44. Law M, Young RJ, Babb JS, et al. Gliomas: predicting time to progression or survival with cerebral blood volume measurements at dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 2008;247:490–98 10.1148/radiol.2472070898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Deike K, Wiestler B, Graf M, et al. Prognostic value of combined visualization of MR diffusion and perfusion maps in glioblastoma. J Neurooncol 2016;126:463–72 10.1007/s11060-015-1982-z [DOI] [PubMed] [Google Scholar]