Abstract
BACKGROUND AND PURPOSE:
Rapid brain MR imaging is often substituted for head CT in multiply imaged patients with shunted hydrocephalus. Fast TSE-T2 sequences are commonly used in these protocols. One limitation of TSE-T2 sequences is the decreased catheter delineation compared with CT. The aim of this study was to compare fast TSE-T2 with rapid SS-GRE sequences in the evaluation of intracranial shunt catheter delineation as part of a rapid nonsedated pediatric brain MR imaging protocol.
MATERIALS AND METHODS:
We evaluated the findings from 179 consecutive patients who underwent routine clinical imaging according to the rapid nonsedated pediatric brain MR imaging protocol. Comparison of the quality of intracranial shunt catheter localization on SS-GRE versus TSE-T2 was performed.
RESULTS:
Of the total of 179 rapid nonsedated pediatric brain MR images that were reviewed, 62 (35%) had an intracranial shunt catheter. The shunt catheter tip was better localized on the SS-GRE than on the TSE-T2 images in 49/62 (79%) of these patients. Of the remaining 13/62 (21%), the TSE-T2 was either better or equivalent in localizing the shunt catheter tip.
CONCLUSIONS:
Our study shows that rapid SS-GRE sequences can provide better delineation of standard intracranial shunt catheters than standard rapid MR imaging protocols containing only fast TSE-T2 sequences.
CT is generally the preferred imaging technique in the assessment of patients with intracranial shunt catheters. Despite the ease of CT scanning, the delayed risks of childhood radiation are not negligible.1–3 The emergence of rapid MR imaging as a substitute for CT has been influenced by the movement to reduce radiation in these multiply scanned patients. Fast TSE-T2 sequences are commonly used in rapid brain MR imaging.4–8 Despite their utility, at least 2 limitations have been described. One is the lack of sensitivity in identification of extra-axial and parenchymal blood products.9,10 The other is decreased catheter delineation compared with CT.8
One possible remedy to the problem is the addition of GRE sequences to supplement the standard single-shot TSE-T2 sequences of rapid nonsedated pediatric brain MR imaging protocols. These sequences are known to be more sensitive to the susceptibility effects of blood products.9,10 To our knowledge, the utility of these sequences in catheter delineation has not been previously reported. Because these sequences are additions to a brain protocol that necessitates a short scanning time, rapid SS-GRE sequences are preferred over the standard GRE sequences with longer TRs.11,12
The aim of this study was to compare fast TSE-T2 imaging with rapid SS-GRE sequences in the evaluation of intracranial shunt catheter delineation as part of a rapid nonsedated pediatric brain MR imaging protocol.
Materials and Methods
The local institutional review board granted this retrospective study an exemption from obtaining informed consent from participants.
We evaluated the findings from 179 consecutive patients who underwent routine clinical imaging according to the rapid nonsedated brain MR imaging protocol.
Imaging Parameters
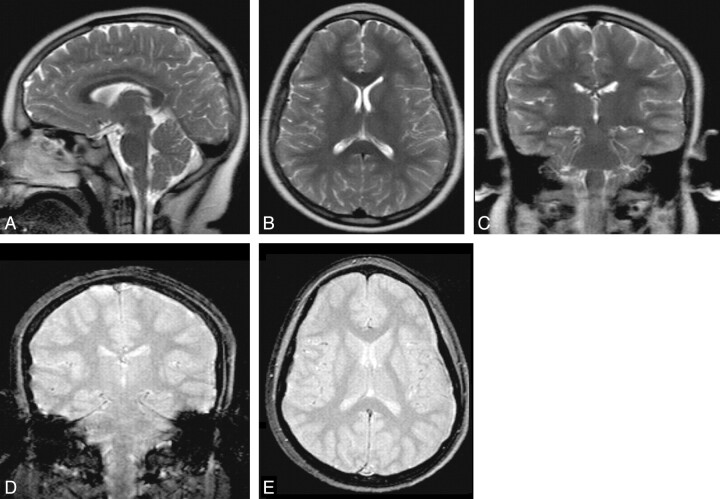
The subjects were imaged by using a 1.5T Intera MR imaging scanner with a 4-channel sensitivity encoding coil (Philips Medical Systems, Best, the Netherlands). They underwent single-shot TSE-T2 imaging (matrix size, 256 × 205; FOV 24; TR/TE/turbo factor, 1500 ms/120 ms/76; 5-mm section thickness; approximately 25 sections; imaging time, approximately 30 seconds) performed in the axial, sagittal, and coronal planes. They also underwent fast postexcitation refocused SS-GRE imaging (matrix size, 256 × 205; FOV 24; TR/TE, 480 ms/14 ms; FA 18°; 5-mm section thickness; approximately 25 sections; imaging time, approximately 30 seconds) performed in the axial and coronal planes. The rationale for adding a coronal SS-GRE sequence in addition to the axial sequence is addressed in the Discussion. The total scanning time was approximately 150 seconds (Fig 1).
Fig 1.
Rapid nonsedated pediatric brain MR imaging protocol. The upper row contains TSE-T2-weighted MR images. The bottom row has fast SS-GRE MR images. The total imaging time was approximately 150 seconds.
Image Assessment
Two pediatric neuroradiologists (J.H.M., T.W.) reviewed the indications and findings of 179 consecutive patients who underwent routine clinical imaging according to our rapid nonsedated brain MR imaging protocol. The images were viewed on a Centricity PACS (GE Healthcare, Milwaukee, Wisconsin). The neuroradiologists viewed the imaging findings and compared the TSE-T2 and SS-GRE imaging for the following: 1) the presence of an intracranial shunt catheter, and 2) the quality of shunt catheter tip localization (better/worse/equally well localized on the GRE sequences compared with the SS-TSE sequences). If localization of the catheter tip was better or equivalent on TSE-T2 compared with SS-GRE sequence, note was made if this difference was due to artifacts.
Assessment of the Clinical Impact of Rapid Nonsedated Brain MR Imaging Protocol Modification
An automated search of the RIS was performed to determine the number of head CT and rapid brain MR imaging orders placed before and after the addition of SS-GRE sequences to the rapid nonsedated brain MR imaging protocol. This search was limited to orders placed by the neurosurgery department for the indication of “hydrocephalus.” Orders placed from the emergency department were excluded because the availability of MR imaging technologists at the time of the order may have influenced the imaging preference. The percentage of total neuroimaging studies that were head CT versus rapid nonsedated brain MR imaging was compared for the 4-month period before and after the protocol addition of SS-GRE sequences.
Results
Rapid Nonsedated Brain MR Imaging Assessment
A total of 179 rapid nonsedated pediatric brain MR images were reviewed. The mean age of the patients at the time of examination was 4 years. The youngest patient was scanned on day 1 of life, and the oldest was 17 years of age. Most patients were younger than 5 years of age (128, 71%). Of the remaining patients, 37 (21%) were 5–10 years of age and 14 (8%) were older than 10 years of age.
There were 62 (35%) patients with an intracranial shunt catheter. The shunt catheter tip was better localized on the SS-GRE than on the TSE-T2 images in 49/62 (79%) of these patients (Figs 2–4). Of the remaining 13/62 (21%), the TSE-T2 sequence was either better or equivalent in localizing the shunt catheter tip. In these patients, there were 2 factors that resulted in greater or equal conspicuity of the catheter tip on TSE-T2 sequences compared with SS-GRE images. In 6 patients, the SS-GRE sequences were nondiagnostic due to patient motion or artifacts from the shunt or reservoir (Fig 5). In the other 7 patients, the degree of ventriculomegaly was at least moderate in severity and the catheter was well positioned within the ventricle. This particular combination of factors provided the most optimal contrast-resolution conditions for localizing the hypointense catheter tip within the hyperintense CSF on TSE-T2 imaging.
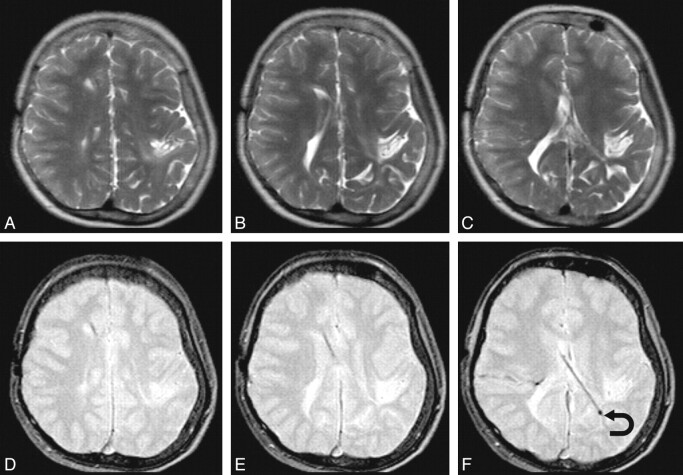
Fig 2.
A representative sample in which SS-GRE is better than TSE-T2 for localizing the ventricular catheter tip. The upper row has TSE-T2-weighted MR images; and the lower row, SS-GRE MR images. The catheter is inserted from a right frontal approach and travels posterolaterally to the left. On TSE-T2-weighted MR images, the exact delineation of the catheter is not certain. On SS-GRE MR images, the entirety of the catheter can be identified with its tip ending in the left posterior periventricular white matter (curved arrow).
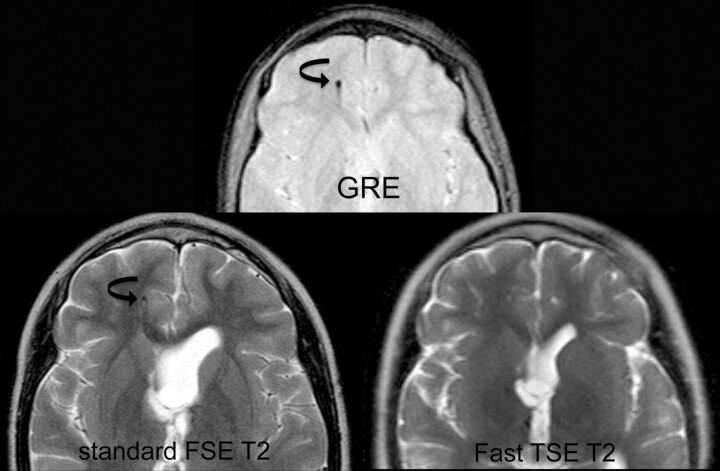
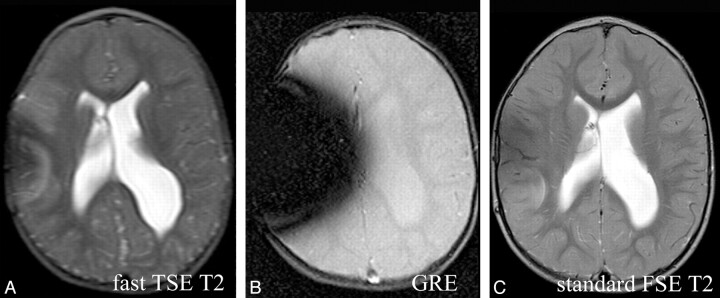
Fig 3.
Comparison of fast SS-GRE MR images with standard T2-weighted MR images for localization of the ventricular catheter tip. On the standard T2-weighted MR image and the SS-GRE MR image, the catheter tip is easily identified in the right frontal white matter (curved arrows). The tip is less conspicuous on the faster TSE T2-weighted MR image.
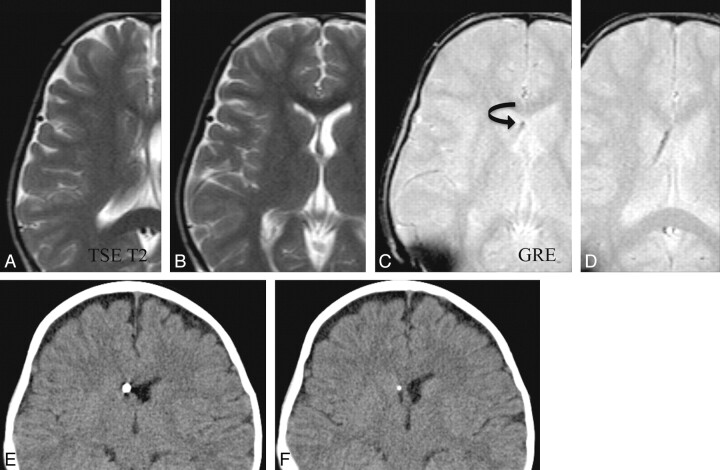
Fig 4.
Comparison of fast SS-GRE MR images with CT for localization of the ventricular catheter tip. The catheter tip is visible within the right frontal horn on CT as well as on SS-GRE MR images (curved arrow). On fast TSE-T2-weighted MR images, the catheter tip is not as readily identified.
Fig 5.
Artifacts secondary to the shunt reservoir. The SS-GRE MR image is the most affected by these artifacts. There are lesser degrees of susceptibility on the fast TSE-T2-weighted and standard T2-weighted MR images, respectively.
Clinical Impact of Rapid Nonsedated Brain MR Imaging Protocol Modification
A RIS search during an 8-month period yielded a total of 159 neuroimaging studies ordered by the neurosurgery department for the indication of “hydrocephalus.” This total did not include those orders placed from the emergency department. This time period consisted of the 4 months before and then the 4 months after the addition of SS-GRE sequences to the rapid nonsedated brain MR imaging protocol. Within the preprotocol modification period, 90 neuroimaging studies were performed. Of these studies, 55 (61%) were head CT and 35 (39%) were rapid nonsedated brain MR imaging protocol. After protocol modification, 79 neuroimaging studies were performed. Of these studies, 42 (53%) were head CT and 35 (47%) were rapid nonsedated brain MR imaging protocol. There was no change in the personnel of the ordering neurosurgery department during this time.
Discussion
Greater awareness of the risks of childhood radiation1–3 and campaigns to reduce radiation exposure in pediatrics (ie, Image Gently)13 have resulted in increased interest in substituting rapid brain MR imaging for head CT in multiply imaged patients with certain neurologic conditions (ie, hydrocephalus). Frequently, single-shot TSE-T2 sequences are used.7,8 These sequences are an acceptable substitute for standard multiecho spin-echo T2 sequences due to their similar image contrast resolution with a much shorter imaging time. In these sequences, a number of consecutive 180° radio-frequency pulses are applied per excitation, resulting in the acquisition of multiple spin-echoes per TR.11,12,14 The drawback to these sequences, however, is that the multiple 180° radio-frequency pulses and the short echo spacing result in a reduced sensitivity to susceptibility effects.9–12,14
GRE images have been shown to be more sensitive in the detection of blood products than fast single-shot TSE-T2 sequences.9,10 Our study shows that GRE sequences can additionally provide improved delineation of standard intracranial catheters compared with TSE-T2 sequences. This increased catheter conspicuity is probably related to a combination of increased contrast resolution (catheter versus background) and the intrinsic properties of the catheter. The fast SS-GRE acquisitions, which were used in our study, were performed in significantly less time (30 seconds for fast SS-GRE versus 4 minutes for standard GRE) without significant difference in contrast-to-noise ratio. Generally the axial images alone were sufficient to localize the catheter tip. The additional images in the coronal plane only occasionally provided increased catheter-localization confidence but were often helpful in evaluating other common abnormalities such as extra-axial convexity blood products.
The reduction in scanning time associated with faster GRE sequences over standard GRE sequences is possible due to the decreased TR of fast SS-GRE sequences (for our protocol 480 ms versus 820 ms). Briefly, standard GRE sequences contain an elongated readout gradient duration (and thus a longer TR) to achieve intrinsic transverse magnetization “spoiling.” Fast GRE sequences either actively “spoil” transverse magnetization or refocus it to contribute to a steady-state formation. Both techniques enable application of a shorter TR compared with standard GRE sequences.9–12,14,15,16 The SS-GRE sequences can be further subdivided into 2 categories when it is remembered that the resultant steady-state precession signal intensity is composed of both FID and spin-echo components. The fast SS-GRE sequence used in our rapid nonsedated pediatric brain protocol is the variety that samples the FID (hence the increased conspicuity of susceptibility effects).16 The vendor-specific names for this sequence are fast-field echo (Philips), fast imaging with steady-state precession (Siemens, Erlangen, Germany), and gradient-recalled acquisition in steady-state (GE Healthcare).15
The increased conspicuity of the intracranial shunts and catheters is likely due to a combination of the susceptibility effects of the intrinsic catheter components and by intravoxel phase dispersion caused by the local magnetic field gradients of the internal tubing structure.17–19 Most intracranial shunts and catheters are composed of a hardened silicone elastomer and are impregnated with barium. Briefly, the silicones are synthetic polymers composed of a repeating silicone-oxygen (siloxane) backbone with organic groups attached through silicone-carbon bonds. There is a variable degree of cross-linking of the siloxane backbone, which determines whether the polymer exists in the gel form, as present in breast implants (10% cross-linking), or in the elastomer form of medical-grade tubing (95% cross-linking).20
Although, to our knowledge, there is no review in the literature of the MR imaging properties of medical grade elastomer tubing, an analysis of the MR imaging properties of silicone elastomer orthopedic tendon spacers does exist.20 In this study, the signal intensity of the imaged silicone elastomer on GRE sequences was at least 70% less than the signal intensity on spin-echo T2 images. These authors hypothesized that the signal-intensity reduction was secondary to local magnetic field gradients within the polymer backbone, resulting in intravoxel phase dispersion of mobile protons traveling along the polymer. Additionally, it is possible that another source of intrinsic susceptibility may come from the diamagnetic effects of the barium with which most catheters are impregnated to provide radio-opacity.
Despite the benefits of the fast GRE sequences that were used in this study, the acquisition parameters did result in a few evident limitations. The relatively long acquisition window (compared with other fast SS-GRE sequences) and its multishot excitation profile (compared with TSE-T2 sequences) resulted in an increased sensitivity to patient motion artifacts. This is not a trivial problem in a protocol designed to image a nonsedated patient who already has a low baseline level of cooperation. The increased susceptibility effect of the shunt catheter reservoir itself was also episodically problematic. This was especially evident when it was located at the same level as the shunt. In our study, increased susceptibility artifacts from the shunt reservoir completely obscured visualization of the intracranial aspect of the catheter in 2 patients. However when these artifactual limitations were not encountered, the fast GRE sequences enabled catheter tip localization equivalent to the criterion standards of CT and standard multishot T2 MR imaging.
The clinical impact of improving the delineation of intracranial shunt catheters is difficult to assess. The clinician responsible for managing an individual patient's shunt catheter often has valuable clinical information regarding the insertion and prior position of the catheter as well as its drainage status. With this knowledge, the radiologist's description of the location of the shunt catheter may not impact clinical decision-making. Given this limitation, it is, however, possible that when taking into account the possible long-term radiation risks of multiple CT examinations, clinicians may increasingly consider substituting rapid MR imaging for CT. Continued technical improvement in rapid MR imaging sensitivity and scanning time may help facilitate its diagnostic ability and increased use.
In an attempt to assess the clinical impact of our rapid MR imaging protocol modification (addition of SS-GRE sequences), a limited review of the ordering preference (rapid MR imaging versus head CT) of the neurosurgery department for patients with hydrocephalus was performed. The small increase in the percentage of rapid MR imaging studies ordered for hydrocephalus (47% versus 39%) after adding the SS-GRE sequences may indicate that the added capabilities of the protocol favorably impacted the confidence of the ordering clinicians (neurosurgery) in substituting rapid MR imaging for CT. This trend, although encouraging, will nonetheless require additional monitoring to assess its validity.
Conclusions
Greater awareness of the risks of childhood radiation and campaigns to reduce radiation exposure in pediatrics (ie, Image Gently) have resulted in increased interest in substituting rapid brain MR imaging for head CT. Fast TSE-T2 sequences are accepted and have proved useful as a substitute. Compared with the criterion standard of head CT, these sequences do, however, have a reduced sensitivity in intracranial catheter localization and blood-product detection.
In our study of rapid nonsedated pediatric brain MR imaging, we have demonstrated that intracranial catheters are more conspicuous on rapid SS-GRE than on fast TSE-T2 sequences. Rapid SS-GRE imaging can be performed in significantly less time than standard gradient sequences. This short scanning time and improved catheter delineation, coupled with improved sensitivity to hemorrhage, make rapid SS-GRE sequences a useful addition to the TSE-T2 sequences in rapid pediatric MR imaging brain protocols.
Abbreviations
- FA
flip angle
- FID
free induction decay
- FSE
fast spin-echo
- GRE
gradient recalled-echo
- RIS
Radiology Information System
- SS-GRE
steady-state gradient recalled-echo
- TSE-T2
T2 turbo spin-echo
Footnotes
Paper previously presented at: Annual Meeting of the Society for Pediatric Radiology, April 21–25, 2009; Carlsbad, California.
References
- 1. Brenner DJ, Elliston, Hall EJ, et al. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol 2001;176:289–96 [DOI] [PubMed] [Google Scholar]
- 2. Verdun FR, Bochud F, Gudinchet F, et al. Quality initiatives: radiation risk—what you should know to tell your patient. Radiographics 2008;28:1807–16 [DOI] [PubMed] [Google Scholar]
- 3. Brenner DJ. Estimating cancer risks from pediatric CT. Pediatr Radiol 2002;32:228–31 [DOI] [PubMed] [Google Scholar]
- 4. Engelbrecht V, Malms J, Kahn T, et al. Fast spin-echo MR imaging of the pediatric brain. Pediatr Radiol 1996;26:259–64 [DOI] [PubMed] [Google Scholar]
- 5. Penzkofer AK, Pfluger T, Pochmann Y, et al. MR imaging of the brain in pediatric patients: diagnostic value of HASTE sequences. AJR Am J Roentgenol 2002;179:509–14 [DOI] [PubMed] [Google Scholar]
- 6. Forbes KP, Pipe JG, Karis JP, et al. Brain imaging in the unsedated patient: comparison of periodically rotated overlapping parallel lines with enhanced reconstruction and single-shot fast spin-echo sequences. AJNR Am J Neuroradiol 2003;24:794–98 [PMC free article] [PubMed] [Google Scholar]
- 7. Ashley WW, McKinstry RC, Leonard JR, et al. Use of rapid-sequence magnetic resonance imaging for the evaluation of hydrocephalus in children. J Neurosurg 2005;103(2 suppl):124–30 [DOI] [PubMed] [Google Scholar]
- 8. Iskandar BJ, Sansone JM, Medow J, et al. The use of quick-brain magnetic resonance imaging in the evaluation of shunt-treated hydrocephalus. J Neurosurg. 2004;101(2 suppl):147–51 [DOI] [PubMed] [Google Scholar]
- 9. Liang L, Korogi Y, Sugahara T, et al. Detection of intracranial hemorrhage with susceptibility-weighted MR sequences. AJNR Am J Neuroradiol 1999;20:1527–34 [PMC free article] [PubMed] [Google Scholar]
- 10. Jones KM, Mulkern RV, Mantello MT. Brain hemorrhage: evaluation with fast spin-echo and conventional dual spin-echo images. Radiology 1992;182:53–58 [DOI] [PubMed] [Google Scholar]
- 11. Frahm J, Haenicke W. Rapid scan techniques. In: Stark DD, Bradley WG. eds. Magnetic Resonance Imaging. 3rd ed. St. Louis: Mosby; 1999:87–124 [Google Scholar]
- 12. Haacke EM, Tkach JA. Fast MR imaging: techniques and clinical applications. AJR Am J Roentgenol 1990;155:951–64 [DOI] [PubMed] [Google Scholar]
- 13. Goske MJ, Applegate KE, Boylan J, et al. The Image Gently campaign: working together to change practice. AJR Am J Roentgenol 2008;190:273–74 [DOI] [PubMed] [Google Scholar]
- 14. Vinitski S, Mitchell DG, Einstein MS, et al. Conventional and fast spin-echo MR imaging: minimizing echo time. J Magn Reson Imaging 1993;3:501–07 [DOI] [PubMed] [Google Scholar]
- 15. Elster A. Gradient-echo MR imaging: techniques and acronyms. Radiology 1993;186:1–8 [DOI] [PubMed] [Google Scholar]
- 16. Chavhan GB, Babyn PS, Jankharia BG, et al. Steady-state MR imaging sequences: physics, classification and clinical applications. Radiographics 2008;28:1147–60 [DOI] [PubMed] [Google Scholar]
- 17. Bos C, Viergever MA, Bakker CJ. On the artifact of a subvoxel susceptibility deviation in spoiled gradient-echo imaging. Magn Reson Med 2003;50:400–04 [DOI] [PubMed] [Google Scholar]
- 18. Smits HF, Bos C, van der Weide R, et al. Interventional MR: vascular applications. Eur Radiol 1999;9:1488–95 [DOI] [PubMed] [Google Scholar]
- 19. Garrido L, Mark JE, Sun CC, et al. NMR characterization of elastomers reinforced with in situ precipitated silica. Macromolecules 1991;24:4067–72 [Google Scholar]
- 20. Kalousdian S, Karlan MS, Williams MA. Silicone elastomer cerebrospinal fluid shunt systems: Council on Scientific Affairs, American Medical Association. Neurosurgery 1998;42:887–92 [DOI] [PubMed] [Google Scholar]