Abstract
BACKGROUND AND PURPOSE:
IIH is a disorder associated with increased intracranial pressure with no clinical, laboratory, or radiologic evidence of an intracranial space-occupying lesion. The aim of this study was to establish ONSD standards of healthy pediatric subjects and compare the normal measurements with those of patients with IIH.
MATERIALS AND METHODS:
One hundred fifteen MR imaging studies of children 4 months to 17 years of age were blinded and reviewed by a pediatric neuroradiologist. A total of 230 optic nerves were measured. Eighty-six MR imaging examinations were performed in apparently healthy subjects. This control group included subjects who underwent MR imaging for various reasons, and their MR imaging findings were interpreted as normal. Twenty-nine MR imaging examinations were performed in patients with documented IIH. The ONSD was measured 1 cm anterior to the optic foramina on an axial T2 sequence. For statistical analysis, both patients and controls were stratified into 4 age groups (I, 0–3 years; II, 3–6 years; III, 6–12 years; IV, 12–18 years).
RESULTS:
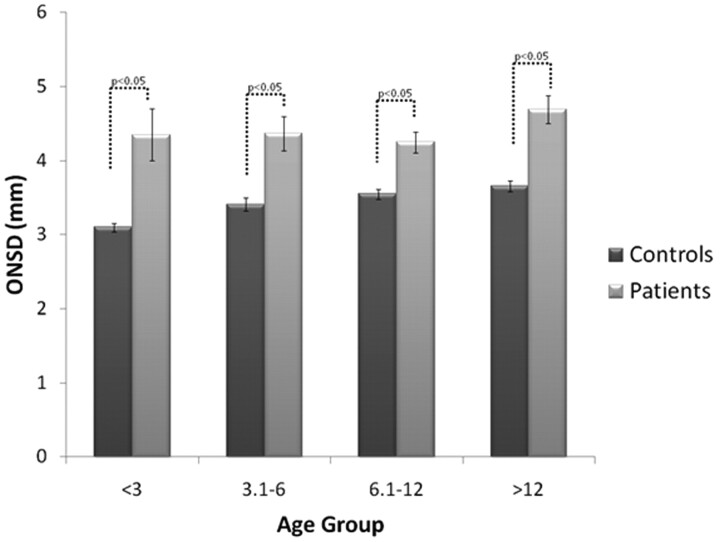
The mean ONSD of the control group in all age groups (I, 3.1 mm; II, 3.41 mm; III, 3.55 mm; IV, 3.56 mm) was significantly smaller than the mean ONSD of patients (I, 4.35 mm; II, 4.37 mm; III, 4.25 mm; IV, 4.69 mm). A positive correlation between age and ONSD (r = 0.414, P < .01) was found in the control group.
CONCLUSIONS:
According to our study, in pediatric patients with IIH, the ONSD is significantly larger than that in healthy controls regardless of age group and sex. This measurement might prove to be an auxiliary tool in the diagnosis of increased intracranial pressure in pediatric patients.
IIH is a disorder in which increased intracranial pressure is measured, with no clinical, laboratory, or radiologic evidence of an intracranial space-occupying lesion.1–3 The diagnosis of IIH is usually established according to the revised modified Dandy criteria (Table 1).4
Table 1:
Revised modified Dandy criteriaa
| Criteria |
|---|
| 1) If symptoms or signs are present, they may only reflect those of generalized intracranial hypertension or papilledema |
| 2) Documented elevated intracranial pressure measured in the lateral decubitus position |
| 3) Normal CSF composition |
| 4) No evidence of hydrocephalus, mass, structural, or vascular lesion on MRI or contrast-enhanced CT for typical patients, and MRI and MR venography for all others |
| 5) No other cause of intracranial hypertension identified |
All of the above criteria should be met in order to make the diagnosis of IIH.
Recent studies have noted that the clinical profile of pediatric patients with IIH differs from the adult type, suggesting that the precipitating factors may be different in prepubertal children.5,6 Unlike the adult distribution, in which there is a strong female predominance,7 IIH in the prepubertal population shows no sex preference. In addition, the outcome in children seems to be better than that in adults, with spontaneous remission more common.8
Recent work dealing with adult populations suggests that there are several subtle neuroradiologic markers that might be helpful when trying to diagnose IIH. For example, flattening of the posterior aspect of the optic globe was proved to be a significant IIH marker.9 Recently, a controlled study emphasized the importance of an increase in the ONSD, as seen on MR imaging. The association between increased intracranial pressure and increasing ONSD was also demonstrated in patients with traumatic brain injury.10
A review of the literature has found very little regarding standardized norms of ONSD on MR imaging for adults,11,12 and no established norms for children. The aim of this study was to establish age-correlated norms for pediatric ONSD and to compare the ONSD of a group of pediatric patients with IIH with a healthy control group. If IIH is consistently associated with an enlarged ONSD, it can be used as an auxiliary noninvasive tool in the investigation of suspected increased intracranial pressure in children.
Materials and Methods
This study was approved by the ethics committee of our institution.
Participants
Control Group.
The control group was composed of children younger than of 18 years of age who underwent MR imaging for various reasons (such as head trauma, seizures, hearing loss, and headaches), and their results were interpreted as normal by an expert pediatric neuroradiologist. Children whose signs and symptoms were similar to those of increased intracranial pressure were excluded from the control group. Additional exclusion criteria were a history of any of the following: intracranial neoplasm, cranial deformity, invasive cranial procedures, and any orbital- or optic-related impairment.
IIH Group.
The IIH group was composed of children younger than of 18 years of age who were diagnosed with IIH according to the revised modified Dandy criteria. Patients with IIH were all diagnosed between the years 2006 and 2009.
Neuroimaging
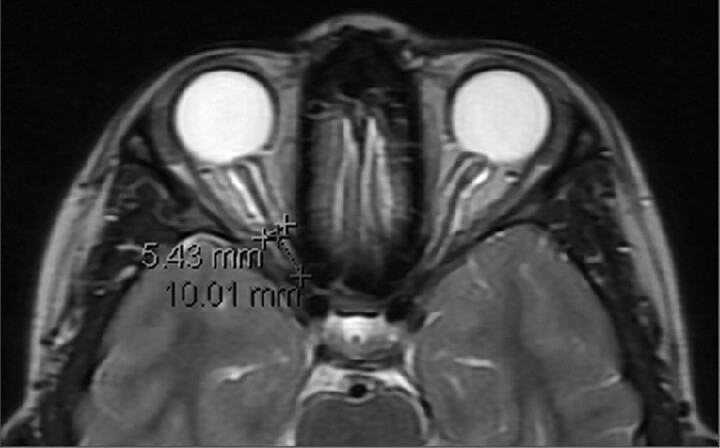
Each of the patients (control and IIH) underwent an MR imaging examination in our institution. MR images (1.5T Signa Excite HDx; GE Healthcare, Milwaukee, Wisconsin) of patients and control subjects were extracted from the records of our medical center. These examinations were routine examinations in which the section thickness varied from 2.5 to 3.5 mm. In some of these examinations, a fat-suppression sequence was available. Examinations in which the optic nerve was not well-demonstrated along with its sheath were excluded. MR imaging studies were blinded and reviewed by a single pediatric neuroradiologist (L.B.S.). In each MR image, after no radiologic evidence of any physiologic cause for intracranial hypertension was verified, optic nerve diameter was measured. The ONSD was always measured at the point 10 mm anterior to the optic foramina on an axial T2 sequence. In cases of a very tortuous optic nerve, this point, no matter where it was spatially (superior or inferior) located, was easily found and measured. We did not encounter an examination in which the tortuosity interfered with measurements. Because the examinations were routine, axial sections were used because they are highly reproducible and are acquired according to a well-defined view. In addition, axial images are routinely used in any basic examination and are most likely to be available for the reviewing radiologist. Coronal images were not always obtained. An example of the measurement method can be seen in Fig 1.
Fig 1.
Example of our measurement method. ONSD is measured 10 mm anterior to the optic foramen. In this example, a distended nerve is measured.
Statistical Analysis
Patients with IIH and control subjects were stratified into 4 age groups: I, 0–3 years; II, 3–6 years; III, 6–12 years; IV, 12–18 years. The mean age in each age group did not differ significantly between patients and controls.
Optic diameters were measured on the MR images in a blinded fashion. The mean width of the optic nerve sheath was calculated separately for the controls and patients with IIH within each age group. The ONSD measurements were analyzed by using the Statistical Package for the Social Sciences, Version 17.0 (SPSS, Chicago, Illinois). Differences between the IIH and control groups were analyzed by using independent-sample t tests for each age group and by using ANCOVA with age as a covariant for the whole study population. The correlation between age, sex, and mean ONSD was analyzed by using the Pearson correlation test.
Results
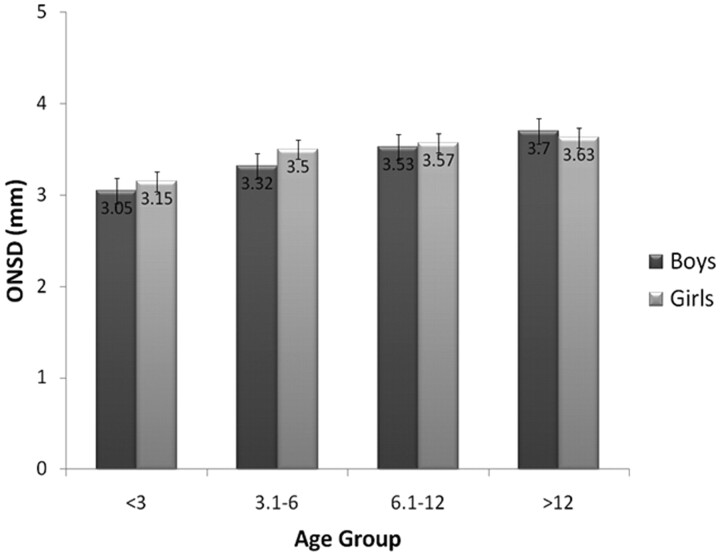
Table 2 summarizes the age, sex, and ONSD data for controls and patients with IIH, organized by age group. No statistical differences were found in the mean ONSD width values between boys and girls in either the control group or the IIH group. A positive correlation between age and ONSD (r = 0.414, P < .01) was found in the control group. The mean ONSD width values of the control group, stratified by age and sex, can be seen in Fig 2.
Table 2:
Study participant data organized by age group
| Control | IIH | |
|---|---|---|
| Total No. | 86 | 29 |
| Sex | M: 41; F: 45 | M: 11; F: 28 |
| Age range | 4 mo-16 yr | 24 mo-17 yr |
| (mean, 8.1 yr; median, 7.7 yr) | (mean, 10.1 yr; median, 11.5 yr) | |
| Group I: 0–3 yr | ||
| No. | 15 | 4 |
| ONSD (avg) | 3.1 mm | 4.35 mm |
| SD | 0.23 mm | 0.7 mm |
| CI (95%) | 0.12 mm | 0.69 mm |
| Group II: 3–6 yr | ||
| No. | 20 | 6 |
| ONSD (avg) | 3.41 mm | 4.37 mm |
| SD | 0.42 mm | 0.57 mm |
| CI (95%) | 0.18 mm | 0.46 mm |
| Group III: 6–12 yr | ||
| No. | 32 | 5 |
| ONSD (avg) | 3.55 mm | 4.25 mm |
| SD | 0.38 mm | 0.32 mm |
| CI (95%) | 0.13 mm | 0.28 mm |
| Group IV: 12–18 yr | ||
| No. | 19 | 14 |
| ONSD (avg) | 3.56 mm | 4.69 mm |
| SD | 0.32 mm | 0.7 mm |
| CI (95%) | 0.14 mm | 0.37 mm |
Note:—avg indicates average; CI, confidence interval.
Fig 2.
Mean ONSD of each age group in our control cohort, stratified by sex. Error bars at the top of each column represent the standard error for that column, ranging from 0.07 to 0.16 mm.
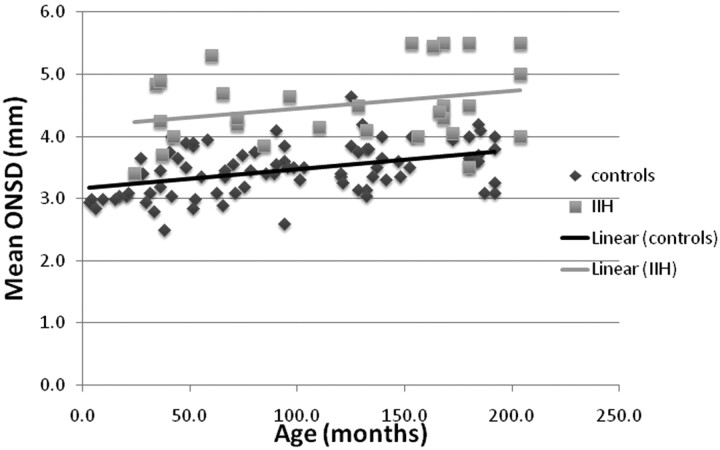
The mean ONSD width values for all IIH age groups was significantly larger than the ONSD width values of the equivalent control age groups (P < .05), as can be seen in Fig 3. When analyzed with an ANCOVA model, the covariate, age, was significantly related to the mean ONSD [F(1,112), P < .01, r = 0.36]. There was also a significant effect of IIH on the mean ONSD after controlling for the effect of age [F(1,112), P < .01]. Scattergraphs and trend lines of both controls and patients with IIH can be seen in Fig 4.
Fig 3.
Mean ONSD width by age group in controls versus patients with IIH.
Fig 4.
Scattergraph and trend lines of the mean ONSD of both controls and patients with IIH.
Discussion
Although the past decade has seen the routine introduction of MR imaging into the neurologic work-up in the pediatric population, age-adjusted norms of the normal ONSD in the pediatric population, usable in the clinical setting, are not available. The only usable MR imaging norms published are of adults.13 To the best of our knowledge, our study presents the first MR imaging age-matched norms developed for ONSD in the pediatric population. These norms may serve as a basis for future inclusion of these measurements in routine clinical evaluation of intracranial pressure. Note that there were no significant differences between the ONSD of boys and girls in all age groups in the control group. This finding excludes sex as a potential confounder for future references. As expected, overall ONSD values correlate positively with age.
Measurements of the optic nerve diameter have always been difficult, due to methodologic problems: the different appearance of the nerve sheath in the various MR imaging sequences, the changing width from anterior to posterior, and so forth. Today, with the use of new volumetric methods and the availability of fat-suppression sequences, such measurements can be rather accurately performed.
An enlarged ONSD, as a reflection of intracranial pressure, is a concept that has been evolving throughout the past 15 years. The rationale behind this concept is derived from the anatomic extension of the subarachnoid space underneath the optic nerve sheath and its connection with cerebral CSF cavities. Increased intracranial pressure is, therefore, believed to cause transmission of force through these spaces, resulting in distention of the ONSD. Most interesting, further support for this theory is suggested in studies showing a similar correlation in the opposite direction (ie, a small ONSD value in the setting of decreased intracranial pressure).14,15
In the pediatric population, Le et al16 tested bedside sonographic assessment of the ONSD in children admitted to an intensive care unit. In their study, ONSD values of 4.0 mm (for children younger than 1 year of age) and 4.5 mm (for older children) were established as the upper limits of the normal diameter. Another US study was performed by Beare et al,17 in which the mean ONSD of pediatric patients with suspected increased intracranial pressure was compared with the ONSD of 30 healthy controls. In this study, an upper limit of 4.2 mm was set. US ONSD measurements are usually taken 3 mm posterior to the optic globe, performed with an axial transbulbar approach.18 This approach utilizes the fact that the proximal retrobulbar area of the nerve is the most prone to distention, is the most echogenic, and is highly reproducible due to its US accessibility. The location of our measurements (10 mm anterior to the optic foramen) was chosen because this location eliminates the possible confounding effect of globe positioning at the time of measurement. The correlation between our measurements and conventional US measurements remains to be examined.
On MR imaging studies, the positive correlation between ONSD width and elevated intracranial pressure has been tested on several occasions when an enlarged ONSD was consistently found to be a strong indicator of increased intracranial pressure in adults. These publications, though highly suggestive, have not been conclusive due to several reasons. Some have examined rather small cohorts19 so that no norms of ONSD could be acquired, while some were ex vivo (cadaveric) in nature.20 Strong support for this theory can also be seen in the work of Geeraerts et al,21 whose study correlated ONSD with invasive intracranial pressure monitoring. Although strongly supporting this theory in adults, these studies were not focused on the pediatric population.
The work published recently by Lim et al,22 dealing with MR imaging features of pediatric patients with IIH, emphasized optic nerve enlargement as one of the important MR imaging markers of IIH, though no measurements were given. Our data show that a simple linear measurement of the optic nerve, if performed 10 mm anterior to the optic foramina on an axial T2 MR imaging section with or without fat suppression, may suggest the presence of increased intracranial pressure at the time of image acquisition. The use of routine imaging performed for different reasons on a basic T2 sequence makes this method applicable to almost any study. The addition of fat suppression makes this measurement easier but is not mandatory. This is a potential noninvasive test to consider because it may provide an alarming sign for increased intracranial pressure.23–25
The data from our study, once placed on a scattergraph, demonstrate some overlap between the subjects with the increased intracranial pressure and controls. Thus ONSD may serve, together with traditional markers of increased intracranial pressure, as a supporting sign and not as a standalone diagnostic tool. MR imaging measurements of the ONSD may be used, if further validated, for the follow-up and monitoring of patients with chronically elevated intracranial pressure.
Conclusions
The establishment of population-based age-adjusted norms is crucial for future reliable use of ONSD in the clinical setting. Our data confirm that optic nerve enlargement may be used as an important warning sign and a noninvasive tool for diagnosis and follow-up of increased intracranial pressure in pediatric patients without space-occupying lesions.
Acknowledgments
We thank Meytal Fischer-Shofty for her assistance with the manuscript and statistics.
ABBREVIATIONS:
- ANCOVA
analysis of covariance
- IIH
idiopathic intracranial hypertension
- ONSD
optic nerve sheath diameter
- US
ultrasonography
References
- 1. Kesler A, Goldhammer Y, Gadoth N. Do men with pseudomotor cerebri share the same characteristics as women? A retrospective review of 141 cases. J Neuroophthalmol 2001; 21: 15– 17 [DOI] [PubMed] [Google Scholar]
- 2. Radhakrishnan K, Ahlskog JE, Cross SA, et al. Idiopathic intracranial hypertension (pseudotumor cerebri): descriptive epidemiology in Rochester, Minn, 1976 to 1990. Arch Neurol 1993; 78– 80 [DOI] [PubMed] [Google Scholar]
- 3. Ahlskog JE, O'Neill BP. Pseudotumor cerebri. Ann Intern Med 1982; 97: 249– 56 [DOI] [PubMed] [Google Scholar]
- 4. Friedman DI, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology 2002; 26 1492– 95 59: [DOI] [PubMed] [Google Scholar]
- 5. Lessell S. Pediatric pseudotumor cerebri (idiopathic intracranial hypertension). Surv Ophthalmol 1992; 37: 155– 66 [DOI] [PubMed] [Google Scholar]
- 6. Baker RS, Baumann RJ, Buncic JR. Idiopathic intracranial hypertension (pseudotumor cerebri) in pediatric patients. Pediatr Neurol 1989; 5: 5– 11 [DOI] [PubMed] [Google Scholar]
- 7. Digre KB, Corbett JJ. Pseudotumor cerebri in men. Arch Neurol 1988; 45: 866– 72 [DOI] [PubMed] [Google Scholar]
- 8. Brodsky MC. Pediaric Neuro-Opthalmology, 2nd ed. New York: Springer, 1996. [Google Scholar]
- 9. Agid R, Farb RI, Willinsky RA, et al. Idiopathic intracranial hypertension: the validity of cross-sectional neuroimaging signs. Neuroradiology 2006; 48: 521– 27 [DOI] [PubMed] [Google Scholar]
- 10. Geeraerts T, Newcombe VF, Coles JP, et al. Use of T2-weighted magnetic resonance imaging of the optic nerve sheath to detect raised intracranial pressure. Crit Care 2008; 12: R114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sheikh M, Abalkhail S, Doi SA, et al. Normal measurement of orbital structures: implications for the assessment of Graves' ophthalmopathy. Australas Radiol 2007; 51: 253– 56 [DOI] [PubMed] [Google Scholar]
- 12. Rohr A, Riedel C, Reimann G, et al. Pseudotumor cerebri: quantitative in-vivo measurements of markers of intracranial hypertension [in German]. Rofo 2008; 180: 884– 90 [DOI] [PubMed] [Google Scholar]
- 13. Ozgen A, Aydingoz U. Normative measurements of orbital structures using MRI. J Comput Assist Tomogr 2000; 24: 493– 96 [DOI] [PubMed] [Google Scholar]
- 14. Rohr A, Jensen U, Riedel C, et al. MR imaging of the optic nerve sheath in patients with craniospinal hypotension. AJNR Am J Neuroradiol 2010; 31: 1752– 57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Watanabe A, Horikoshi T, Uchida M, et al. Decreased diameter of the optic nerve sheath associated with CSF hypovolemia. AJNR Am J Neuroradiol 2008; 29: 863– 64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Le A, Hoehn ME, Smith ME, et al. Bedside sonographic measurement of optic nerve sheath diameter as a predictor of increased intracranial pressure in children. Ann Emerg Med 2009; 53: 785– 91 [DOI] [PubMed] [Google Scholar]
- 17. Beare NA, Kampondeni S, Glover SJ, et al. Detection of raised intracranial pressure by ultrasound measurement of optic nerve sheath diameter in African children. Trop Med Int Health 2008; 13: 1400– 04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Soldatos T, Chatzimichail K, Papathanasiou M, et al. Optic nerve sonography: a new window for the non-invasive evaluation of intracranial pressure in brain injury. Emerg Med J 2009; 26: 630– 34 [DOI] [PubMed] [Google Scholar]
- 19. Watanabe A, Kinouchi H, Horikoshi T, et al. Effect of intracranial pressure on the diameter of the optic nerve sheath. J Neurosurg 2008; 109: 255– 58 [DOI] [PubMed] [Google Scholar]
- 20. Steinborn M, Fiegler J, Kraus V, et al. High resolution ultrasound and magnetic resonance imaging of the optic nerve and the optic nerve sheath: anatomic correlation and clinical importance. Ultraschall Med 2011; 32: 608– 13 [DOI] [PubMed] [Google Scholar]
- 21. Geeraerts T, Merceron S, Benhamou D, et al. Non-invasive assessment of intracranial pressure using ocular sonography in neurocritical care patients. Intensive Care Med 2008; 34: 2062– 67 [DOI] [PubMed] [Google Scholar]
- 22. Lim MJ, Pushparajah K, Jan W, et al. Magnetic resonance imaging changes in idiopathic intracranial hypertension in children. J Child Neurol 2010; 25: 294– 99 [DOI] [PubMed] [Google Scholar]
- 23. Salgarello T, Tamburrelli C, Falsini B, et al. Optic nerve diameters and perimetric thresholds in idiopathic intracranial hypertension. Br J Ophthalmol 1996; 80: 509– 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kesler A, Yaffe D, Shapira M, et al. Optic nerve sheath enlargement and reversal of optic nerve head in pseudotumor cerebri [in Hebrew]. Harefuah 1996; 130: 457– 59, 503 [PubMed] [Google Scholar]
- 25. Gass A, Barker GJ, Riordan-Eva P, et al. MRI of the optic nerve in benign intracranial hypertension. Neuroradiology 1996; 38: 769– 73 [DOI] [PubMed] [Google Scholar]