Figure 7: De Novo Ceramide Biosynthesis Mediates Systolic Dysfunction in KLF5 Transgenic Mice –
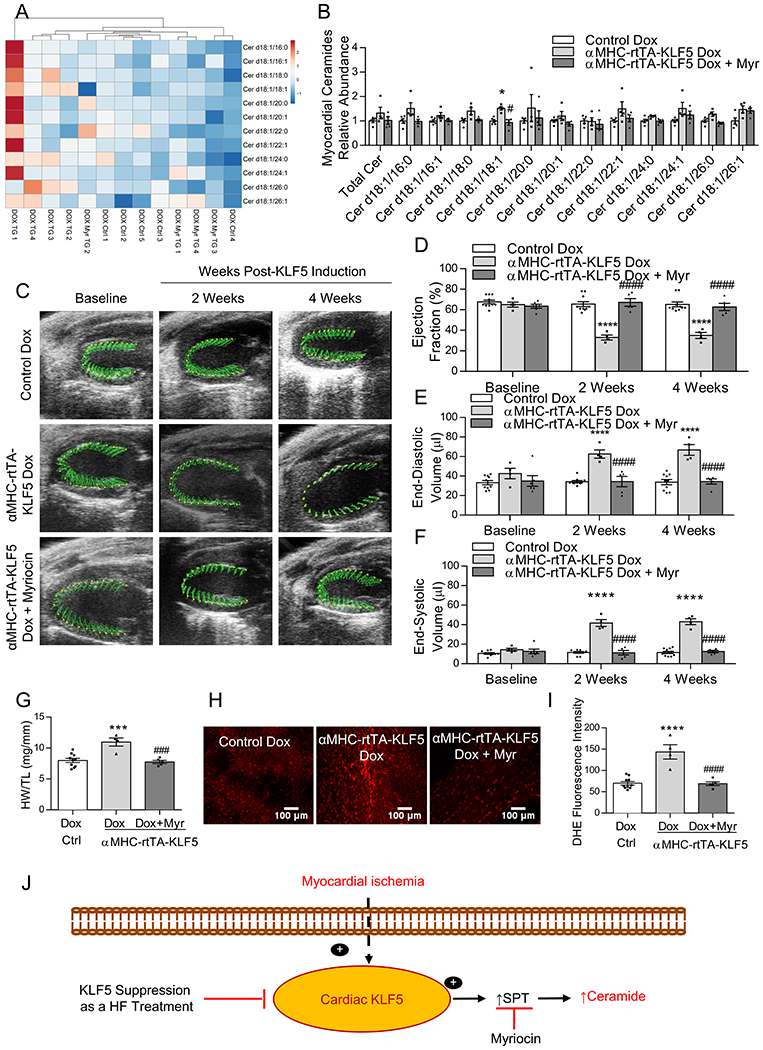
Hierarchical clustering based upon myocardial ceramides (A), and quantification of total myocardial ceramide levels and individual ceramide family members in control mice, αMHC-rtTA-KLF5 mice, and αMHC-rtTA-KLF5 mice treated with myriocin by LC-MS/MS relative to control mice (B). *p<0.0042 vs control, #p<0.0042 vs αMHC-rtTA-KLF5 by ANOVA with Tukey HSD and α corrected to 0.0042 for multiple tests for each lipid family member. n=5 control, n=4 αMHC-rtTA-KLF5, n=4 αMHC-rtTA-KLF5 + myriocin. Lipidomic data is available in the Supplemental excel file 1. Representative echocardiography images (C) and quantification of ejection fraction (D), end-diastolic volume (E), and end-systolic volume (F) in control mice, αMHC-rtTA-KLF5 mice, and αMHC-rtTA-KLF5 mice treated with myriocin at baseline, 2-weeks, and 4-weeks post-induction. n=10 control, n=4 αMHC-rtTA-KLF5, n=5 αMHC-rtTA-KLF5 + myriocin for echocardiography analysis. *p<0.05, ****p<0.0001 vs control; #p<0.05, ####p<0.0001 vs αMHC-rtTA-KLF5 by two-way ANOVA with Tukey HSD. Heart weight normalized to tibia length (G), DHE staining of live myocardium (H), and quantification of DHE fluorescence (I) in control mice, αMHC-rtTA-KLF5 mice, and αMHC-rtTA-KLF5 mice treated with myriocin. n=10 control, n=4 αMHC-rtTA-KLF5, n=5 αMHC-rtTA-KLF5 + myriocin. *p<0.05, ***p<0.001, ****p<0.0001 vs control, ###p<0.001, ####p<0.0001 vs αMHC-rtTA-KLF5 by one-way ANOVA with Tukey HSD. Proposed model depicting the mechanism through which KLF5 regulates ceramide biosynthesis in ischemic heart failure (J).
