Abstract
BACKGROUND AND PURPOSE:
For patients with ICH, knowing the rate of CT contrast extravasation may provide insight into the pathophysiology of hematoma expansion. This study assessed whether the PCT-derived PS can measure different rates of CT contrast extravasation for admission CTA spot signs, PCCT, PCL, and regions without extravasation in patients with ICH.
MATERIALS AND METHODS:
CT was performed at admission and at 24 hours for 16 patients with ICH with/without contrast extravasation seen on CTA and PCCT. PCT-PS was measured at admission. The Wilcoxon rank sum test with a Bonferroni correction was used to compare PS values from the following regions of interest: 1) spot sign lesions only (9 foci), 2) PCL lesions only (9 foci), 3) hematoma excluding extravasation, 4) regions contralateral to extravasation, 5) hematoma in patients without extravasation, and 6) an area contralateral to that in 5. Additionally, hematoma expansion was determined at 24 hours defined by NCCT.
RESULTS:
PS was 6.5 ± 1.60 mL · min−1 × (100 g)−1, 0.95 ± 0.39 mL · min−1 × (100 g)−1, 0.12 ± 0.39 mL · min−1 × (100 g)−1, 0.26 ± 0.09 mL · min−1 × (100 g)−1, 0.38 ± 0.26 mL · min−1 × (100 g)−1, and 0.09 ± 0.32 mL · min−1 × (100 g)−1 for the following: 1) spot sign lesions only (9 foci), 2) PCL lesions only (9 foci), 3) hematoma excluding extravasation, 4) regions contralateral to extravasation, 5) hematoma in patients without extravasation, and 6) an area contralateral to that in 5. PS values from spot sign lesions and PCL lesions were significantly different from each other and all other regions, respectively (P < .05). Hematoma volume increased from 34.1 ± 41.0 mL to 40.2 ± 46.1 mL in extravasation-positive patients and decreased from 19.8 ± 31.8 mL to 17.4 ± 27.3 mL in extravasation-negative patients.
CONCLUSIONS:
The PCT-PS parameter measures a higher rate of contrast extravasation for CTA spot sign lesions compared with PCL lesions and hematoma. Early extravasation was associated with hematoma expansion.
Primary ICH accounts for ∼20% of all strokes and leads to clinical deterioration.1 During the first 3 hours of symptom onset, early hematoma growth is seen in 18%–38% of patients with ICH, reducing to 11% thereafter.2–4 Early hematoma expansion is associated with poorer neurologic outcome and increased mortality.5 The cause of early hematoma expansion is unknown, but secondary vessel injury and perihematomal ischemia have been implicated.6 Prevention or reduction of secondary bleeding is important when dealing with ICH. Recently, administration of recombinant factor VIIa resulted in the reduction of hematoma expansion, without improvement in clinical outcome7; however, earlier drug administration has been shown to increase symptom reversal in a small subgroup of patients.8
CT imaging is considered the technique of choice for the acute investigation of ICH. Extravasation is demonstrated on CTA and PCCT in approximately 40%–50% of patients.9,10 Several imaging studies have demonstrated an association between early contrast extravasation and hematoma expansion.10–13 A recent study demonstrated that foci of contrast leakage not discernable during the arterial phase of a CTA may be detected on PCCT (PCL).14 A further study demonstrated that delayed CTA detected other areas of contrast extravasation not seen on an early CTA.15 In both studies, sensitivity to hematoma expansion improved when delayed images were considered: It is reasonable to assume that a slower rate of contrast extravasation in PCL accounts for the lack of CTA detection, though this has not been shown. Furthermore, the etiology and pathophysiologic significance of the various manifestations of contrast extravasation remain unknown. However, given the association between the risk of hematoma expansion and poor outcome with the number and attenuation of extravasation lesions, it is likely that more rapid extravasation, such as that seen on arterial phase CTA, may confirm the highest risk.13
PCT-derived PS is a novel method of measuring the rate of contrast extravasation from the intra- to extravascular compartment.16 Knowing the rate of contrast extravasation may provide insight into the pathophysiology of hematoma expansion by identifying the contributing target abnormality. A clinical technique that quantifies this rate provides objective assessment of hematoma expansion risk rather than the qualitative approach currently used. Such information may become increasingly important as novel ICH treatments are developed that will target specific lesions, such as focused sonography coagulation.17
Using the PCT-PS parameter, we sought to measure the rate of contrast extravasation for CTA spot sign lesions and PCCT-PCL lesions, respectively. PS values from these 2 abnormalities were compared with PS values from hematoma volumes, excluding any extravasation; hematoma volumes in patients without extravasation; and contralateral normal tissue. We hypothesized that the rate of contrast extravasation would be highest in CTA spot sign lesions compared with PCL lesions and hematoma regions without extravasation.
Materials and Methods
Patient Cohort
With approval by the local institutional ethics review board, 16 consecutive patients with ICH presenting during a 6-month period at a tertiary stroke center underwent initial NCCT and CTA on admission. The imaging was reviewed acutely with the patient on the CT table, by 1 of 2 attending neuroradiologists (5 years' experience). If ICH was identified, a PCCT scan and a PCT study were performed regardless of the result of contrast extravasation in CTA. Spot sign and PCL extravasation were identified retrospectively by the presence of contrast extravasation on CTA or PCCT, respectively. Of 16 screened patients, images in 7 demonstrated contrast extravasation. Nine foci of contrast extravasation were present on each of the CTA and PCCT images, respectively, for a total of 18 regions.
Image Acquisition
CT was performed on a 64-section CT scanner (Lightspeed Plus and VCT; GE Healthcare, Milwaukee, Wisconsin) at admission and 24 hours after presentation in all patients. The standard ICH protocol includes an NCCT head scan followed by CTA and PCCT. Pre- and postcontrast head imaging was performed from the skull base to the vertex with the following parameters: 120 kV(peak), 340 mA, 4 × 5 mm collimation, 1 s/rotation, and a table speed of 15 mm/rotation. CTA studies were acquired from C6 to the vertex in the helical half scan mode with the following parameters: 0.7 mL/kg of contrast (to a maximum of 90 mL through an antecubital vein via at least an 18- or 20-gauge angiocatheter), 5- to 10-second delay, 120 kV(p), 270 mA, 1 s/rotation, 1.25-mm section thickness at 0.625-mm intervals, and table speed of 3.75 mm/rotation. The PCT study comprised 2 phases.
The first phase was a 45-second continuous (cine) scan reconstructed at 0.5-second intervals to produce a series of 90 sequential images for each of the 8 sections, covering a total of 40 mm from the basal ganglia to the lateral ventricles. The second phase collected images covering the same 8 sections during an additional 90 seconds, immediately after the first phase at intervals of 15 seconds. Scan parameters for both phases were the following: 80 kV(p), 190 mA, 8 × 5 mm collimation, and 1 s/rotation. The dose-length products for NCCT, CTA (intracranial only), and PCT components were 1073, 592, and 1804 mGy × cm, respectively. If one assumed a conversion factor of 0.0021 mSv per mGy × cm, the effective dose was 7.3 mSV. Iodinated contrast agent at a dose of 0.5 mL/kg (maximum, 50 mL) was injected 3 to 5 seconds before the start of the first phase at a rate of 4 mL · s−1. A 5-minute postprocessing time is the same as that previously reported.18 The follow-up consisted of an NCCT only. All images were viewed on an Impax 4.5 PACS workstation (Agfa-Gevaert, Mortsel, Belgium).
Image Analysis
PCT 4 software (GE Healthcare) was used to calculate parametric maps of PS. Arterial input and venous output functions were obtained from the ipsilateral anterior cerebral artery and from the superior sagittal sinus, respectively (Figs 1 and 2). Partial volume averaging was corrected by multiplying the arterial input function by the ratio of the area of the venous time-attenuation curve to that of the arterial input function.19 Maps were calculated by deconvolution of the arterial input curve and tissue curves from 2 × 2 pixel blocks of CT images by using the adiabatic approximation to the J-W model.20
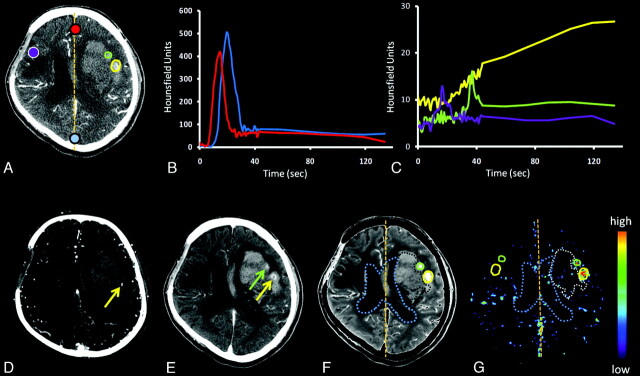
Fig 1.
A, Baseline contrast-enhanced source PCT image. B, Corresponding time attenuation curves for the artery (red) and vein (blue). C, Time attenuation curves for the spot sign (yellow), PCL (green), and contralateral region of interest (purple). D, CTA with the spot sign visible (yellow arrow). E, PCCT shows PCL (green arrow) and the spot sign with extravasation (yellow arrow). F and G, PWI (F) and PS (G) map. Regions of interest encircle the contrast extravasation (spot sign and PCL) on the PWI and the entire hematoma, excluding extravasation and overlap with ventricles (intraventricular hemorrhage). Regions of interest are superimposed on the PS map and are reflected about the midline.
Fig 2.
A, Baseline contrast-enhanced source PCT image. B, Corresponding time attenuation curve for the artery (red) and vein (blue). C, TDC for the region of interest within the hematoma (yellow) and contralateral region of interest (green). D and E, CTA and PCCT with no visible contrast extravasation within the hematoma. F and G, PWI (F) and PS map (G). A region of interest encircles the hematoma on the PWI. The hematoma region of interest is superimposed on the PS map, excluding overlap with ventricles, and is reflected about the midline.
PS, by definition, is the unidirectional rate of contrast extravasation from the intravascular to the extravascular space through a disrupted blood-brain barrier.21 Extravasation of contrast material leads to prolonged enhancement of the tissue beyond the intravascular (first) phase, which can only be properly characterized by a 2-phase PCT study as previously described.18 Parametric maps were analyzed by 1 author (C.D.D, 4 years' experience) by using custom software (IDL, Version 6.1; RSI, Boulder, Colorado). Extravasation, observed on CTA and/or PCCT, was located on inherently coregistered PWI from the PCT protocol. Four regions of interest were superimposed onto coregistered PS functional maps for extravasation-positive patients: They were areas of focal contrast extravasation on 1) the location of a CTA spot sign lesion, and/or 2) the location of the PCL lesion, 3) the entire hematoma excluding both extravasation and overlap with ventricles (IVH), and 4) mirror regions contralateral to extravasation regions defined in 1 and 2 (Fig 1).
For extravasation-negative patients, regions of interest were placed on the following areas: 1) the entire hematoma volume excluding overlap with ventricles, and 2) mirror regions contralateral to the region defined in 1 (Fig 2). Regions of interest were superimposed onto CBF, CBV, and PS functional maps; pixels with values of CBF >100 mL × min−1 × (100 g)−1 and CBV >8 mL × (100 g)−1 were excluded.22 Hematoma volumes at presentation and follow-up were calculated blinded to extravasation status by using Medical Image Processing, Analysis and Visualization (Center for Information Technology, National Institutes of Health, Bethesda, Maryland). Hematoma expansion was defined as an increased volume of >6 mL or 30%.11,23 IVH was not considered in the definition of hematoma expansion.
Statistical Analysis
All analyses were performed with the Statistical Package for the Social Sciences for Windows (Version 16, SPSS, Chicago, Illinois), and all datasets were checked for normality with the Shapiro-Wilk calculation. The Wilcoxon rank sum test with a Bonferroni correction was used to compare all PS values. Significance was defined as P < .05. Average PS values were compared for the following: 1) spot sign lesions only (9 foci), 2) PCL lesions only (9 foci), 3) hematoma excluding extravasation, 4) regions contralateral to extravasation, 5) hematoma in patients without extravasation, and 6) an area contralateral to that in 5. Unpaired t tests were used to compare differences in age, time to CTA, average arterial pressure, international normalized ratio, partial thromboplastin time, NIHSS score, and glucose score, respectively. With the Wilcoxon rank sum test with a Bonferroni correction, the average hematoma volume at follow-up (24 hours) was compared with admission hematoma volume within extravasation-positive and -negative groups, respectively.
Results
PS data were acquired for 16 patients with ICH (9 men and 7 women). Their demographics are listed in Table 1. Of the 7 patients with extravasation on the admission CTA and/or PCCT imaging, 3 patients had both spot sign and PCL lesions, 3 patients had only PCL lesions, and 1 patient had only a spot sign lesion. Nine patients did not present with extravasation on CTA or PCCT. One extravasation-positive patient received recombinant factor VIIa as an off-label treatment. The average PS was 6.5 ± 1.60 mL · min−1 × (100 g)−1, 0.95 ± 0.39 mL · min−1 × (100 g)−1, 0.12 ± 0.39 mL · min−1 × (100 g)−1, 0.26 ± 0.09 mL · min−1 × (100 g)−1, 0.38 ± 0.26 mL · min−1 × (100 g)−1, and 0.09 ± 0.32 mL · min−1 × (100 g)−1 for the following: 1) spot sign lesions only (9 foci), 2) PCL lesions only (9 foci), 3) hematoma excluding extravasation, 4) regions contralateral to extravasation, 5) hematoma in patients without extravasation, and 6) an area contralateral to that in 5. PS values from spot sign lesions and PCL lesions were significantly different from each other and all other regions of interest (P < .05; Table 2). Average absolute or percentage hematoma volume increased from 34.1 ± 41.0 to 40.2 ± 46.1 mL or 27.8% in extravasation-positive patients. In extravasation-negative patients, absolute or percentage volume decreased from 19.8 ± 31.8 mL to 17.4 ± 27.3 mL, or −1.5% (Table 3).
Table 1:
Demographics for 16 patients with ICHa
| Extravasation-Positive | Extravasation-Negative | P Value | |
|---|---|---|---|
| Age (yr) | 73.83 ± 22.96 | 63.80 ± 17.13 | .23 |
| Time to CTA (min) | 234.50 ± 384.95 | 131.00 ± 46.96 | .74 |
| MAP | 142.72 ± 27.12 | 160.25 ± 51.02 | .69 |
| INR | 1.15 ± 0.32 | 1.05 ± 0.11 | .16 |
| PTT | 33.23 ± 4.78 | 31.54 ± 4.24 | .15 |
| Glucose (mmol/L) | 6.57 ± 1.78 | 6.85 ± 1.41 | .31 |
| NIHSS (median) (range) | 15 (5–23) | 11 (2–16) | .37 |
Note:—MAP indicates average arterial pressure; INR, international normalized ratio; PTT, partial thromoboplastin time.
Patients are dichotomized into extravasation-positive (n = 7) and -negative (n = 9) groups. Unpaired t tests are used to compare the data.
Table 2:
PS data for 16 patients with ICHa
| Extravasation-Positive |
Extravasation-Negative |
|||||
|---|---|---|---|---|---|---|
| Spot Sign | PCL Lesions | Hematoma without Extravasation | Contralateral to Extravasation | Hematoma | Contralateral ROI | |
| lesions (9 foci) | (9 foci) | ROI | ROI | |||
| Mean PS | 6.5 ± 1.6b | 0.95 ± 0.39b | 0.12 ± 0.39 | 0.26 ± 0.09 | 0.38 ± 0.26 | 0.09 ± 0.32 |
| Median PS | 6.69 | 0.87 | 0.15 | 0.29 | 0.42 | 0.07 |
| InterQ range | 5.31–7.36 | 0.67–0.98 | 0.06–0.34 | 0.25–0.31 | 0.14–0.59 | 0.03–0.40 |
Note:—InterQ indicates interquartile; ROI, region of interest.
PS values (milliliter × minute−1 × [100 g]−1) for patients with (n = 7) and without (n = 9) contrast extravasation. Subgroups are based on ROI location. Wilcoxon rank sum tests with a Bonferroni correction were used to compare data.
Significantly different (P < .05) from all other subgroup mean PS values.
Table 3:
Hematoma volume changes for 16 patients with ICHa
| Initial Volume (cm3) | Final Volume, (cm3) | Volume Change (cm3) | Volume Change (%) | P Value | |
|---|---|---|---|---|---|
| Extravasation-positive | 34.00 ± 41.00 | 40.24 ± 46.15 | 5.68 ± 13.45 | 27.84 ± 74.70 | .86 |
| Extravasation-negative | 19.89 ± 31.86 | 17.47 ± 27.31 | −2.42 ± 4.70 | −1.56 ± 35.23 | .95 |
Patients are dichotomized into extravasation-positive (n = 7) and -negative (n = 9) groups. Initial and final hematoma volumes were measured at admission and 24 hours, respectively. Wilcoxon rank sum tests with a Bonferroni correction were used to compare initial and final hematoma volumes within both groups, respectively.
Discussion
Early CT contrast extravasation (CTA spot sign and PCL) is an important predictor of ICH evolution.10,12,14,15 Size and attenuation of contrast on admission CTA reflect the rate of contrast accumulation and have recently been shown to be 2 of 3 factors associated with hematoma expansion and patient outcome.15 This study uses PCT-derived PS measures to quantify the differential rates of contrast leakage in contrast extravasation abnormalities observed on admission CTA or PCCT. A technique such as PCT that quantifies the contrast leakage rate may better define which patients with extravasation are at highest risk for expansion. Hematoma volume expansion occurred more frequently in patients with extravasation, who also tended to have worse clinical outcomes.
Although the pathophysiologic significance of the CTA spot sign and the PCCT-PCL lesion is uncertain, we have previously suggested that the former may represent the primary causative abnormality, whereas the latter may represent secondary vessel injury due to hematoma shearing.24 Nonetheless, PCL appears to be significant for hematoma growth compared with patients with no extravasation; however, in a recent study, 40% of patients with the PCL lesion did not demonstrate hematoma expansion.14 As such, the clinical effect of PCL is certainly overshadowed by the presence of the CTA spot sign; Wada et al13 showed that only 4% of patients without hematoma expansion were spot sign−positive.
The rate of contrast extravasation is an important consideration when assessing the sensitivity of modalities being used. Slower rates of contrast accumulation will be more difficult to detect with early scan techniques if insufficient contrast attenuation has accumulated. Two recent studies reported higher rates of extravasation detection by using a delayed CTA acquisition (median 113-second delay following initial CTA) or PCCT (typically performed 3–5 minutes following contrast injection).14,15 Herein, the detection of 9 extravasation lesions on PCCT, not present on early CTA, is consistent with these accounts. Most important, however, all PCL lesions could be visualized as increased PS on postprocessed PCT functional maps. By virtue of a dynamic acquisition lasting 2 minutes after contrast injection, PCT appears more sensitive in assessing different contrast-leakage rates than either CTA or PCCT; however, this remains to be confirmed in a larger clinical study. PCT has the advantage of not requiring a precontrast decision between early and late scanning. Furthermore, the overall dose of PCT is similar to that in a NCCT and avoids radiosensitive structures such as the thyroid and orbits.
Quantification of the rate of leakage provides a unique opportunity to objectively study the effect of the rate of contrast extravasation on hematoma expansion, for which the CTA spot sign is currently a surrogate marker. The high PS values obtained in patients with the CTA spot sign emphasize the rapid rate of contrast extravasation, reinforcing the need for a rapid and efficient treatment. Herein, we calculated the PS with the Johnson-Wilson distributed parameter model, which accounts for the following: 1) the “gradual” leakage of contrast as it travels from the arterial input to the venous output, and 2) the clearance (backflux) of contrast from the interstitial space into the vascular space. Another tracer kinetic model for the calculation of PS is the Patlak model, which, unlike the Johnson-Wilson model, uses compartments to represent the vascular and interstitial spaces.25,26 As such, it is required that both contrast arrival and leakage through the brain vasculature are instantaneous, as opposed to a gradual finite transit. Thus, the Patlak model is not valid under conditions in which the arterial contrast concentration is changing rapidly, as seen during the vascular (first) phase of our data acquisition. This is exemplified by the initial region of nonlinearity observed in the Patlak plot, when the first-pass data are included.27 Whereas the Johnson-Wilson model can be used to measure PS, CBV, and CBF simultaneously, the Patlak model provides estimates of PS and CBV but not CBF, which has to be estimated separately by using another calculation/model.26 The Patlak model also assumes that there is no clearance (backflux) of contrast from the interstitial space into the vascular space. As a result, PS measured with the Johnson-Wilson model, which accounts for backflux, will be higher than that derived from the Patlak model.
Several limitations of this study need to be discussed. First, the case series is limited by a small sample size. For practical reasons, patients were recruited by supervising neuroradiologists during daytime admissions. Although difficult to achieve given the relatively low incidence of ICH admissions and lower incidence of contrast extravasation, these results will require validation from larger patient cohorts. Second, the influence of systemic and local features such as blood pressure, hematoma location, mass effect, and shift, respectively, on the rate of extravasation and hematoma growth is currently unknown; this drawback limits the prediction of potential hematoma volume. Similarly, the interaction of different patterns of extravasation on each other when coexisting in the same hematoma is uncertain. Third, due to the concurrence of PCCT-PCL and the CTA spot sign in some patients, we were unable to compare hematoma expansion in patients harboring only PCL or a spot sign. Fourth, the CTA, PCCT, and PCT imaging was separated by ∼10–15 minutes, during which time the PS could conceivably change. Finally, the PS may be underestimated because varying amounts of blood, which has a lower Hounsfield unit than CT contrast, can leak into the extravascular space during the PCT acquisition.
Conclusions
The PCT-PS parameter measures different rates of CT contrast extravasation for patients with ICH with/without the CTA spot sign and/or PCL lesions. This information could be used to guide hemostatic treatment during the acute stroke stage.
Abbreviations
- CBF
cerebral blood flow
- CBV
cerebral blood volume
- CTA
CT angiography
- ICH
intracerebral hemorrhage
- IVH
intraventricular hemorrhage
- NCCT
non-contrast CT
- NIHSS
National Institutes of Health Stroke Scale
- PCCT
post–contrast-enhanced CT
- PCL
postcontrast leakage
- PCT
perfusion CT
- PS
permeability surface area product
- PWI
perfusion-weighted imaging
Footnotes
Disclosures: Ting-Yim Lee: Research Support (including provision of equipment or materials): GE Healthcare; Details: Research grant; Other Financial Interests: GE Healthcare; Details: licensing of CT Perfusion software.
References
- 1. Broderick JP, Brott TG, Tomsick T, et al. Ultra-early evaluation of intracerebral hemorrhage. J Neurosurg 1990;72:195–99 [DOI] [PubMed] [Google Scholar]
- 2. Kazui S, Naritomi H, Yamamoto H, et al. Enlargement of spontaneous intracerebral hemorrhage: incidence and time course. Stroke 1996;27:1783–87 [DOI] [PubMed] [Google Scholar]
- 3. Fujii Y, Takeuchi S, Sasaki O, et al. Multivariate analysis of predictors of hematoma enlargement in spontaneous intracerebral hemorrhage. Stroke 1998;29:1160–66 [DOI] [PubMed] [Google Scholar]
- 4. Brott T, Broderick J, Kothari R, et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke 1997;28:1–5 [DOI] [PubMed] [Google Scholar]
- 5. Broderick JP, Brott TG, Duldner JE, et al. Volume of intracerebral hemorrhage: a powerful and easy-to-use predictor of 30-day mortality. Stroke 1993;24:987–93 [DOI] [PubMed] [Google Scholar]
- 6. Kidwell CS, Saver JL, Mattiello J, et al. Diffusion-perfusion MR evaluation of perihematomal injury in hyperacute intracerebral hemorrhage. Neurology 2001;57:1611–17 [DOI] [PubMed] [Google Scholar]
- 7. Mayer SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2008;358:2127–37 [DOI] [PubMed] [Google Scholar]
- 8. Mayer SA, Davis SM, Skolnick BE, et al. Can a subset of intracerebral hemorrhage patients benefit from hemostatic therapy with recombinant activated factor VII? Stroke 2009;40:833–40 [DOI] [PubMed] [Google Scholar]
- 9. Becker KJ, Baxter AB, Bybee HM, et al. Extravasation of radiographic contrast is an independent predictor of death in primary intracerebral hemorrhage. Stroke 1999;30:2025–32 [DOI] [PubMed] [Google Scholar]
- 10. Goldstein JN, Fazen LE, Snider R, et al. Contrast extravasation on CT angiography predicts hematoma expansion in intracerebral hemorrhage. Neurology 2007;68:889–94 [DOI] [PubMed] [Google Scholar]
- 11. Wada R, Aviv RI, Fox AJ, et al. CT angiography “spot sign” predicts hematoma expansion in acute intracerebral hemorrhage. Stroke 2007;38:1257–62 [DOI] [PubMed] [Google Scholar]
- 12. Kim J, Smith A, Hemphill JC, et al. Contrast extravasation on CT predicts mortality in primary intracerebral hemorrhage. AJNR Am J Neuroradiol 2008;29:520–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Delgado Almandoz JE, Yoo AJ, Stone MJ, et al. The spot sign score in primary intracerebral hemorrhage identifies patients at highest risk of in-hospital mortality and poor outcome among survivors. Stroke 2010;41:54–60. Epub 2009 Nov 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ederies A, Demchuk A, Chia T, et al. Postcontrast CT extravasation is associated with hematoma expansion in CTA spot negative patients. Stroke 2009;40:1672–76 [DOI] [PubMed] [Google Scholar]
- 15. Delgado Almandoz JE, Yoo AJ, Stone MJ, et al. Systematic characterization of the computed tomography angiography spot sign in primary intracerebral hemorrhage identifies patients at highest risk for hematoma expansion: the spot sign score. Stroke 2009;40:2994–3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. St Lawrence KS, Lee TY. An adiabatic approximation to the tissue homogeneity model for water exchange in the brain. II. Experimental validation. J Cereb Blood Flow Metab 1998;18:1378–85 [DOI] [PubMed] [Google Scholar]
- 17. Connor CW, Hynynen K. Patterns of thermal deposition in the skull during transcranial focused ultrasound surgery. IEEE Trans Biomed Eng 2004;51:1693–706 [DOI] [PubMed] [Google Scholar]
- 18. Aviv RI, d'Esterre CD, Murphy BD, et al. Hemorrhagic transformation of ischemic stroke: prediction with CT perfusion. Radiology 2009;250:867–77 [DOI] [PubMed] [Google Scholar]
- 19. Cenic A, Nabavi DG, Craen RA, et al. Dynamic CT measurement of cerebral blood flow: a validation study. AJNR Am J Neuroradiol 1999;20:63–73 [PubMed] [Google Scholar]
- 20. Lee TY, Purdie TG, Stewart E. CT imaging of angiogenesis. Q J Nucl Med 2003;47:171–87 [PubMed] [Google Scholar]
- 21. St Lawrence KS, Lee TY. An adiabatic approximation to the tissue homogeneity model for water exchange in the brain. I. Theoretical derivation. J Cereb Blood Flow Metab 1998;18:1365–77 [DOI] [PubMed] [Google Scholar]
- 22. Kudo K, Terae S, Katoh C, et al. Quantitative cerebral blood flow measurement with dynamic perfusion CT using the vascular-pixel elimination method: comparison with H2(15)O positron emission tomography. AJNR Am J Neuroradiol 2003;24:419–26 [PMC free article] [PubMed] [Google Scholar]
- 23. Chang EF, Meeker M, Holland MC. Acute traumatic intraparenchymal hemorrhage: risk factors for progression in the early post-injury period. Neurosurgery 2006;58:647–56 [DOI] [PubMed] [Google Scholar]
- 24. Thompson AL, Kosior JC, Gladstone DJ, et al. Defining the CT angiography ‘spot sign' in primary intracerebral hemorrhage. Can J Neurol Sci 2009;36:456–61 [DOI] [PubMed] [Google Scholar]
- 25. Hom J, Dankbaar JW, Soares BP, et al. Blood-brain barrier permeability assessed by perfusion CT predicts symptomatic hemorrhagic transformation and malignant edema in acute ischemic stroke. AJNR Am J Neuroradiol 2011;32:41–48. Epub 2010 Oct 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin K, Kazmi KS, Law M, et al. Measuring elevated microvascular permeability and predicting hemorrhagic transformation in acute ischemic stroke using first-pass dynamic perfusion CT imaging. AJNR Am J Neuroradiol 2007;28:1292–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dankbaar JW, Hom J, Schneider T, et al. Dynamic perfusion CT assessment of the blood-brain barrier permeability: first pass versus delayed acquisition. AJNR Am J Neuroradiol 2008;29:1671–76 [DOI] [PMC free article] [PubMed] [Google Scholar]