Fig. 1. Structural variations among native and unnatural cystobactamids and structure comparison with albicidin.
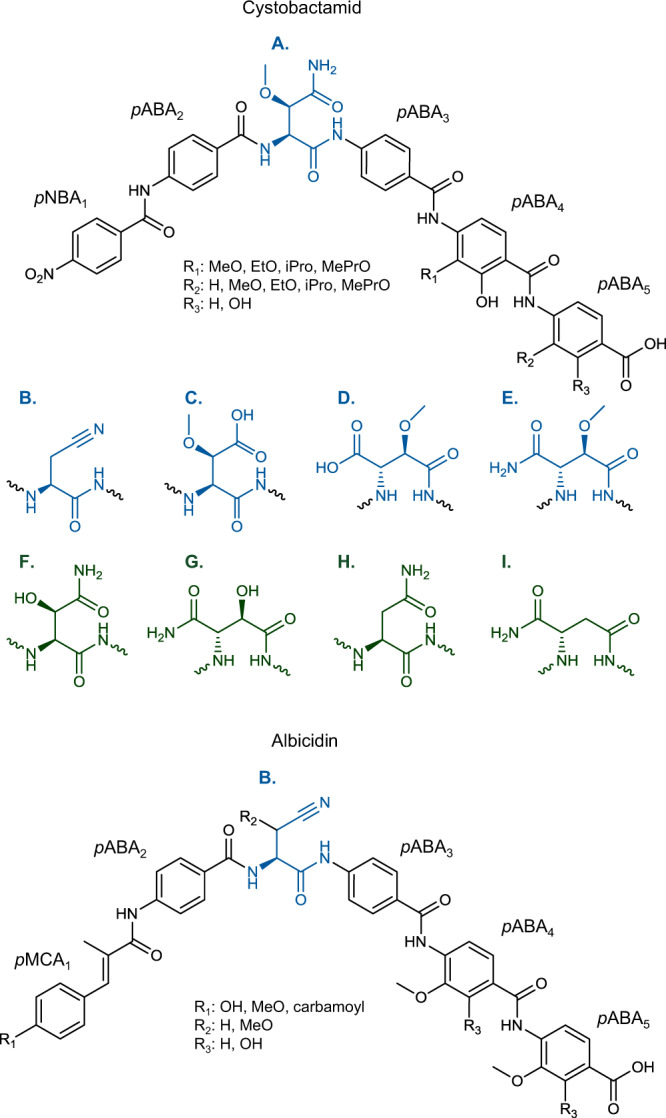
para-Nitrobenzoic acid (pNBA) and para-aminobenzoic acid (pABA) with possible substitutions (R1, R2, R3) are shown in black. Different linker moieties of natural cystobactamids (shown in blue; linker (A)–(E)) and unnatural cystobactamids (shown in green; linker (F)–(I); Table 1): (A) β-methoxy-l-asparagine, (B) β-cyano-l-alanine, (C) β-methoxy-l-aspartate, (D) α-methoxy-l-isoaspartate, (E) α-methoxy-l-isoasparagine, (F) β-hydroxy-l-asparagine, (G) α-hydroxy-l-isoasparagine, (H) l-asparagine, (I) l-isoasparagine. The scheme was adapted from Hüttel et al.2. The stereochemistry of the linker moiety is based on the assignment by Planke et al.44. Albicidin carries an N-terminal para-methylcoumaric acid (pMCA1), two pABAs, two substituted pABAs, and a (possibly modified) β-cyano-l-alanine (B) or β-methoxy-l-asparagine (A) linker. Different possible substitutions (R1, R2, R3) are given.
