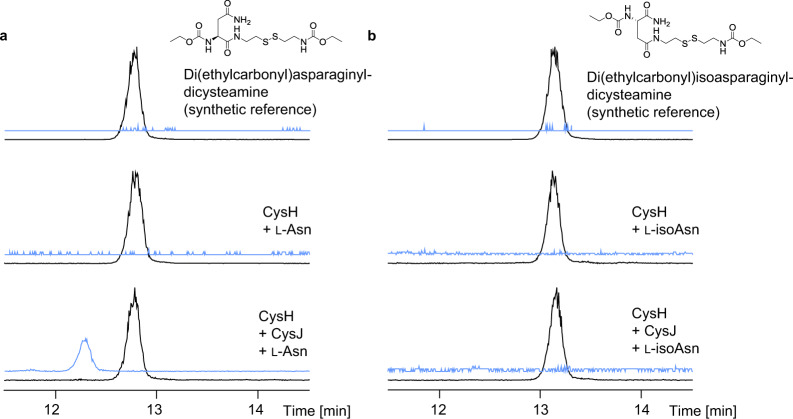
Fig. 4. Hydroxylation of CysH-bound l-asparagine by CysJ.
HPLC-MS analysis of cysteamine-unloaded and derivatized substrate from the CysH protein. EICs 411.1 m/z [M + H]+ are shown in black and EICs 427.1 m/z [M + H]+ (Di(ethylcarbonyl)-hydroxy-l-(iso)asparaginyl-dicysteamine) are shown in blue. a Di(ethylcarbonyl)-l-asparaginyl-dicysteamine synthetic reference. CysH-bound l-asparagine unloaded and derivatized showed the same retention time as the synthetic reference. Incubation of CysH with CysJ and l-asparagine lead to hydroxylation of l-asparagine and different retention times of the unloaded substrate compared to the reference. b Di(ethylcarbonyl)-l-isoasparaginyl-dicysteamine synthetic reference. CysH-bound l-isoasparagine unloaded and derivatized showed the same retention time as the synthetic reference. No hydroxylation occurred upon the addition of CysJ.