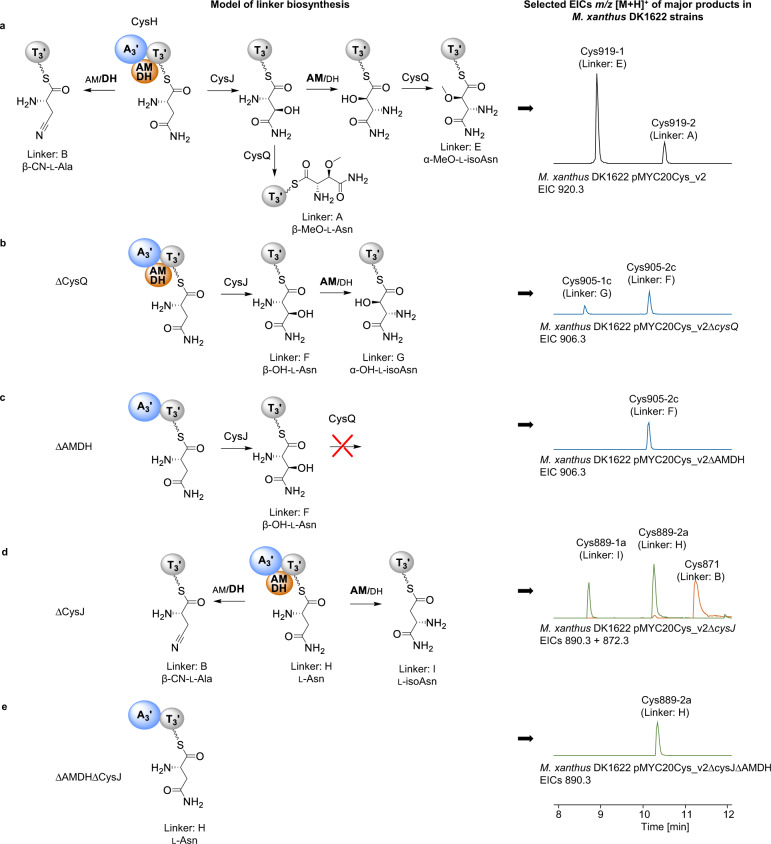
Fig. 5. Summary of biosynthesis pathways for cystobactamid linkers.
a Hydroxylation of CysH-bound l-asparagine by CysJ with subsequent isomerization by AMDH plus O-methylation by CysQ leading to α-methoxy-l-isoasparagine (E linker); O-methylation without isomerization leads to β-methoxy-l-asparagine (A linker); direct dehydration by AMDH domain leads to the formation of β-cyano-l-alanine (B linker). EIC 920.3 [M + H]+ (black) shows the production of Cys919-1 (E linker) and Cys919-2 (A linker) in M. xanthus DK1622 pMYC20Cys_v2. b Absence/deletion of CysQ leads to the formation of β-hydroxy-l-asparagine (F linker) or α-hydroxy-l-isoasparagine (G linker) after isomerization by AMDH. EIC 906.3 [M + H]+ (blue) shows the production of Cys905-1c (G linker) and Cys905-2c (F linker), which lack one methyl group in the linker compared to Cys919 (−14 Da shift). c Deletion of the AMDH domain leads to hydroxylation of CysH-bound l-asparagine by CysJ, but O-methylation by CysQ does not occur (only formation of β-hydroxy-l-asparagine/production of Cys905-2c). d Absence/deletion of CysJ leads to the formation of l-asparagine (H linker), l-isoasparagine (I linker), or β-cyano-l-alanine (B linker). Overlay of EIC 890.3 [M + H]+ (green) and EIC 872.3 [M + H]+ (orange) shows the production of Cys889-1a (I linker), Cys889-2a (H linker) and Cys871 (B linker) e Deletion of CysJ and the AMDH domain prevents any modification of asparagine leading only to the production of Cys889-2a. Blue sphere: adenylation domain; gray sphere: thiolation domain; orange sphere: AMDH domain; red cross: nonfunctional pathway.