Abstract
BACKGROUND AND PURPOSE:
The clinical relevance of improved detection of cerebral microbleeds by using advanced-versus-conventional MR imaging techniques remains uncertain. As part of the population-based Rotterdam Scan Study, we compared whether participants whose microbleeds were only demonstrated on a high-resolution MR imaging sequence differed with respect to risk profile and risk of new microbleeds from participants whose microbleeds were also depicted on a conventional MR imaging sequence.
MATERIALS AND METHODS:
Two hundred participants (mean age, 79.2 years) underwent both conventional 2D T2*-weighted MR imaging and high-resolution 3D T2*-weighted MR imaging at 1.5T. Vascular risk factors, APOE allele status, and markers of small vessel disease and risk of incident microbleeds were compared for microbleed status by using logistic regression models adjusted for age and sex.
RESULTS:
There were no significant associations between any of the factors and microbleed presence in participants whose microbleeds were only demonstrated on a high-resolution MR imaging sequence. However, the estimates in these participants were more similar to those in participants whose microbleeds were also depicted on a conventional MR imaging sequence than to those in participants without microbleeds. Moreover, significantly more participants whose microbleeds were only demonstrated on high-resolution MR imaging developed new CMBs during follow-up compared with participants without CMBs (25.0% versus 5.9%; OR, 5.98; 95% CI, 1.35–26.49).
CONCLUSIONS:
Improved detection of microbleeds may contribute to more accurate identification of persons with underlying small-vessel pathology in the general elderly population. Further studies are needed to replicate these findings and firmly establish the role of improved detection of CMBs in the identification of persons with vasculopathy.
There is increasing evidence that CMBs mark underlying vasculopathy1 and that their presence may have important prognostic implications.2 Previously, it has been reported that the choice of MR imaging parameters and a higher field strength and the use of advanced postprocessing (eg, SWI) substantially affect detection of CMBs.3–5 Recently, data from patients in memory clinics suggested that though SWI detected more CMBs in more patients compared with conventional T2*-weighted GRE imaging, clinical relevance in terms of associations with vascular risk factors or radiologic markers of small-vessel disease was not increased.6 The risk profile of participants who were classified as having microbleeds based only on SWI, however, remained unclear. This group is of interest because it is uncertain whether hypointensities detected only on high-end sequences and not on conventional imaging are either artifacts or very small microbleeds that may not have the same relevance as larger microbleeds.6,7 In addition to studying the risk profile in this specific group, an ideal proof of concept would be to investigate their risk of developing new microbleeds with time.
Using data from the population-based Rotterdam Scan Study, we compared whether participants whose microbleeds were depicted only on a high-resolution MR imaging sequence differed with respect to vascular risk factors, APOE allele status, and/or markers of small vessel disease from participants whose microbleeds were also demonstrated on a conventional sequence. Moreover, we examined whether these participants had an increased risk of incident microbleeds during a 3-year follow-up period.
Materials and Methods
Participants
In 2006, 254 participants from a larger population-based prospective cohort—the Rotterdam Study—were eligible for the present brain MR imaging study.4 Institutional review board approval was obtained. In total, 207 (81.5%) agreed to participate and gave informed consent. Complete and technically adequate MR imaging data were obtained in 200 participants (mean age, 79.2 years; range, 69.7–96.7 years).4 From 2009 to 2010, these participants were re-invited for follow-up MR imaging. Of the 200 participants at baseline, 42 were not eligible to participate in the second MR imaging examination (deceased, n = 28; new MR imaging contraindication [eg, pacemaker], n = 3; institutionalized, n = 9; untraceable, n = 2). Of 158 eligible participants, follow-up MR imaging examinations were acquired in 108 participants (54% of participants at baseline; mean interval between the 2 MR imaging assessments, 3.0 years).
Rating of Cerebral Microbleeds
Imaging was performed on a 1.5T MR imaging scanner (GE Healthcare, Milwaukee, Wisconsin). In 2006, in all participants, conventional 2D T2*-weighted imaging (TR = 775 ms; TE = 20 ms; flip angle = 25°; section thickness = 5 mm) and higher resolution 3D T2*-weighted imaging (TR = 45 ms; TE = 31 ms; flip angle = 13°; parallel imaging acceleration factor = 2; section thickness = 1.6 mm, zero padded to 0.8 mm) were performed. Follow-up imaging was conducted from 2009 to 2010 on the same 1.5T scanner by using the above-described 3D T2*-weighted sequence.
Both sequences performed at baseline were reviewed in the acquired resolution according to international guidelines.8 In brief, 2 trained raters recorded the presence, number, and location of cerebral microbleeds. Reviewers were blinded to the other sequence and to all clinical information, and scans were read in random order (interobserver reliabilities, κ = 0.80–0.90). All studies with potential microbleeds were reviewed for confirmation by an experienced neuroradiologist. At this time, the T1-weighted images were used additionally to differentiate microbleeds from calcification in the ventricle or sulcal vessels. Differences between 2D and 3D images were assessed in a side-by-side comparison. Assessment of incident microbleeds on 3D T2*-weighted images was performed in a side-by-side comparison blinded to the time point of the scans.9
Assessment of Risk Profile
Cardiovascular risk factors were assessed by interview and by laboratory and physical examination as previously described.1 APOE genotyping was performed on coded genomic deoxyribonucleic acid samples. Lacunar infarcts were scored as present or absent on FLAIR, proton-density–weighted, and T1-weighted MR imaging sequences (all viewed together) by the same raters who had scored CMBs. Lacunar infarcts were defined as focal lesions ≥3 mm and <15 mm with the same signal characteristics as CSF on all sequences and (when located supratentorially) with a hyperintense rim on the FLAIR sequence.1 White matter lesion volume was quantified with a validated fully automated tissue-classification technique.10
Statistical Analysis
Participants were categorized at baseline as having “no CMBs” (no microbleeds on either 2D or 3D T2*GRE), “CMBs on 3D but not 2D” (≥1 microbleed on 3D T2*GRE but no microbleeds on the 2D T2*GRE), and “CMBs on 2D and 3D ” (≥1 microbleed on both 2D T2*GRE and 3D T2*GRE sequences). We reported the prevalence of vascular risk factors, APOE allele status, and markers of cerebral small-vessel disease among these different categories. Furthermore, we assessed associations of these correlates with the prevalence of CMBs in these categories by using binary logistic regression models with risk factors as covariates and the presence of microbleeds (no/yes) as dependent variables. All models were adjusted for age and sex. In addition, we assessed, for each category, the risk of incident CMBs with binary logistic regressions adjusted for age, sex, and scan interval.
Results
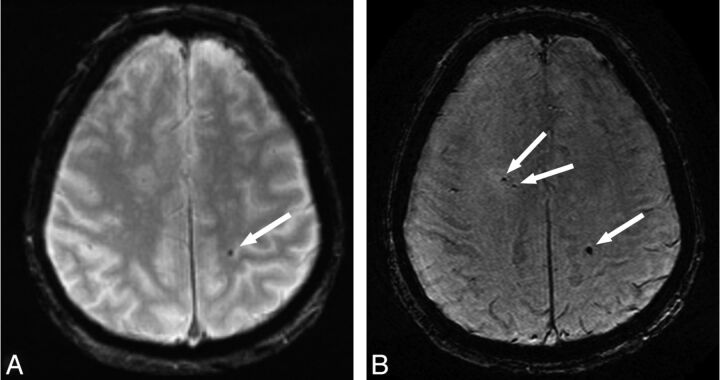
CMBs were detected on the 2D T2*-weighted images of 42 (21%) participants and on the 3D T2*-weighted images of 71 (35.5%) participants (difference, P < .001). This resulted in 29 participants in the category CMBs on 3D, but not 2D, and 42 participants in the category CMBs on 2D and 3D. No CMBs were visualized on the 2D T2*-weighted images that were not detected on the 3D T2*-weighted images. The median number of microbleeds was 1.0 (mean, 7.6; interquartile range, 1.0–4.0) on the 2D T2*-weighted images, whereas this number was 2.5 (mean, 9.9; interquartile range, 1.0–9.5) on the 3D T2*-weighted images (Fig 1).4 The distribution of microbleeds in lobar, deep, or infratentorial locations was similar between both sequences.4
Fig 1.
Axial MR images obtained with a conventional 2D T2*-weighted sequence (A) and a high-resolution 3D T2*-weighted sequence (B) in the same participant. More cerebral microbleeds (arrows) are visible on the high-resolution image compared with the conventional image.
The Table shows the association of vascular risk factors, APOE allele status, and markers of small-vessel disease with the presence of microbleeds within the aforementioned categories of CMB detection levels. There were no significant associations between any of these factors and microbleed presence in the CMBs on 3D but not 2D category. However, the estimates for sex, blood pressure, and serum cholesterol of participants in the CMBs on 3D but not 2D category were remarkably similar to those in the CMBs on 2D and 3D category, whereas estimates for hypertension, APOE ε4 carriership, presence of lacunar infarcts, and white matter lesion volume of this CMBs on 3D but not 2D category were between those of participants without microbleeds and persons with microbleeds on both sequences (Table).
Prevalence of vascular risk factors, APOE allele status, and markers of small-vessel disease according to microbleed statusa
| Variables | No CMBsb (n = 129) |
CMBs on 3D But Not 2Dc (n = 29) |
CMBs on 2D and 3Dd (n = 42) |
|||
|---|---|---|---|---|---|---|
| % or mean ± SD | % or mean ± SD | OR (95% CI)e | % or mean ± SD | OR (95% CI)e | ||
| Age, years | 78.8 ± 5.8 | 79.2 ± 6.3 | 1.01 (0.95–1.09) | 80.6 ± 6.3 | 1.05 (0.99–1.11) | |
| Men | 43.4 | 51.7 | 1.40 (0.63–3.15) | 50.0 | 1.27 (0.63–2.57) | |
| Systolic blood pressuref (mm Hg) | 145.3 ± 19.6 | 150.8 ± 21.4 | 1.42 (0.91–2.22) | 152.8 ± 21.2 | 1.43 (0.98–2.08) | |
| Diastolic blood pressuref (mm Hg) | 75.6 ± 10.0 | 79.0 ± 10.5 | 1.48 (0.94–2.32) | 76.5 ± 9.4 | 1.33 (0.88–2.00) | |
| Hypertension (WHO grades 2 and 3) | 22.6 | 42.9 | 1.48 (0.52–4.23) | 38.5 | 2.44 (0.81–7.31) | |
| Smoking (ever) | 74.8 | 72.4 | 0.79 (0.31–2.06) | 82.9 | 1.59 (0.61–4.11) | |
| Serum total cholesterolg (mmol/L) | 5.60 ± 1.05 | 5.52 ± 1.07 | 0.92 (0.57–1.50) | 5.56 ± 0.94 | 0.93 (0.60–1.43) | |
| APOE ε4 carrier, versus ε3/ε3 | 22.9 | 34.6 | 1.74 (0.67–4.52) | 42.1 | 2.72 (1.17–6.35) | |
| Lacunar infarct on MRI | 9.3 | 31.0 | 2.70 (0.82–8.94) | 23.8 | 2.92 (1.03–8.30) | |
| White matter lesions on MRIh (mL) | 7.3 (3.6–15.1) | 9.3 (4.0–26.8) | 1.35 (0.87–2.09) | 9.9 (6.3–30.8) | 1.52 (1.04–2.22) | |
Note:—WHO indicates World Health Organization.
All values represent %, mean ± SD, or age- and sex-adjusted (when applicable) ORs with 95% CI.
Participants without microbleeds on either 2D or 3D T2*GRE.
Participants with ≥1 microbleed on 3D T2*GRE but without microbleeds on the 2D T2*GRE.
Participants with ≥1 microbleed on both 2D T2*GRE and 3D T2*GRE sequences.
Odds ratios compared with the odds of the “no CMBs” category.
Additionally adjusted for the use of blood pressure–lowering medication.
Additionally adjusted for the use of lipid-lowering drugs.
Median with interquartile range and natural-log transformed values used in odds ratios.
Moreover, significantly more participants in the CMBs on 3D but not 2D category developed new CMBs during follow-up compared with participants without CMBs (25.0% versus 5.9%; OR, 5.98; 95% CI, 1.35–26.49). For persons in the CMBs on 2D and 3D category, this risk was approximately 2-fold higher (40%; OR, 12.77; 95% CI, 3.08–52.93).
Discussion
During the past 5 years, several studies have demonstrated that improved MR imaging techniques (eg, higher field strength, higher resolution, or use of advanced postprocessing) yield a higher detection rate of cerebral microbleeds compared with conventional T2*-weighted MR imaging.3–5 It is debated, however, whether hypointensities detected only on “high-end” sequences have the same significance as microbleeds detected on conventional MR imaging. Recently, Goos et al6 concluded that though more CMBs in more patients were detected by using more advanced imaging (SWI) compared with conventional imaging techniques, this improved detection had no improved clinical relevance in terms of associations with vascular risk factors or radiologic markers of small-vessel disease. They hypothesized that larger CMBs readily detectable on conventional imaging may serve as a small-vessel disease marker, whereas perhaps smaller lesions, visible on SWI only, may only do so to a lesser extent.6 We performed a similar comparison between a conventional and a higher resolution T2*-weighted MR imaging sequence, and we found that participants who were rated positive for microbleed presence only on the high-resolution sequence were more alike in risk profile to persons who had microbleeds on both imaging sequences than to persons without microbleeds. Due to small numbers, however, most associations of risk factors with microbleed presence did not reach significance in the present dataset. It was previously shown in a larger cohort, however, that all of these risk factors were significantly related to microbleed presence.1,11 We found that risk estimates in persons who were rated positive for microbleeds only on high-resolution imaging were either remarkably similar or slightly lower compared with persons with microbleeds on both image sequences. This finding supports the hypothesis that there is less severe underlying vasculopathy in this group compared with participants who had CMBs depicted on both image sets, but again, power issues need to be considered.
In the present study, of the 200 participants with a baseline MR imaging examination, 108 persons had a complete and reliable follow-up MR imaging examination (54%). This rate is comparable with other large population-based studies, (eg, the Cardiovascular Health Study, in which 58% of participants underwent follow-up MR imaging after 5 years; and the Atherosclerosis Risk in Communities Study, in which 61% of participants underwent follow-up MR imaging after 10 years),12,13 especially in the light of the high mean age (79.2 years at baseline) of our study participants. Selective drop-out may have influenced our results regarding the incidence of microbleeds. People who participated in follow-up MR imaging were younger and healthier compared with those who refused a second MR imaging scan or, in particular, were ineligible for MR imaging. This may have led us to underestimate the true incidence of microbleeds in the population at large. Despite this, participants rated positive for microbleed presence only on the high-resolution sequence had a significantly increased risk of incident microbleeds during a 3-year time interval, further supporting true underlying vasculopathy in this group. Because follow-up imaging was performed by using only the high-resolution MR imaging, we were not able to compare the incidence of microbleeds on the conventional-versus-higher-resolution T2*-weighted MR imaging sequence and, therefore, cannot exclude the possibility that the incident microbleeds detected on high-resolution images may be related to a less severe or different form of vasculopathy compared with incident microbleeds depicted on conventional T2*-weighted images.
We agree with Goos et al6 that CMBs may be considered the “tip of the iceberg ” for underlying vascular pathology and that more sensitive MR imaging sequences—or, for example, higher field strengths—may reveal the larger iceberg. Our results suggest, however, that the larger “tip ” that we identify by using an advanced MR imaging sequence may provide clinically relevant information in community-dwelling elderly.
Conclusions
Improved detection of CMBs may contribute to more accurate identification of persons with underlying vascular pathology in the general elderly population, which is increasingly important in clinical practice, as well as in clinical trials. Further studies are needed to replicate these findings and firmly establish the role of improved detection of CMBs in the identification of persons with vasculopathy.
ABBREVIATIONS:
- APOE
apolipoprotein E
- CI
confidence interval
- CMB
cerebral microbleed
- GRE
gradient recalled-echo
- OR
odds ratio
Footnotes
The Rotterdam Study is supported by the Erasmus MC University Medical Center and Erasmus University Rotterdam; Netherlands Organization for Scientific Research; Netherlands Organization for Health Research and Development; Research Institute for Diseases in the Elderly; Netherlands Genomics Initiative; Ministry of Education, Culture and Science; Ministry of Health, Welfare and Sports; European Commission, and Municipality of Rotterdam. This study was further financially supported by NWO grants 948-00-010 and 918-46-615.
The funding sources had no role in the design or conduct of the study, data collection, data analysis, data interpretation, or in writing or approval of this work.
Disclosures: Meike W. Vernooij—RELATED: Grants/Grants Pending: grant from the Alzheimer's Association (NIRG-09–13168). Mohammad Ikram—UNRELATED: Grants/Grants Pending: Netherlands Heart Foundation, International Parkinson Fonds.
References
- 1. Vernooij MW, van der Lugt A, Ikram MA, et al. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology 2008; 70: 1208–14 [DOI] [PubMed] [Google Scholar]
- 2. Bokura H, Saika R, Yamaguchi T, et al. Microbleeds are associated with subsequent hemorrhagic and ischemic stroke in healthy elderly individuals. Stroke 2011; 42: 1867–71 [DOI] [PubMed] [Google Scholar]
- 3. Nandigam RN, Viswanathan A, Delgado P, et al. MR imaging detection of cerebral microbleeds: effect of susceptibility-weighted imaging, section thickness, and field strength. AJNR Am J Neuroradiol 2009; 30: 338–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vernooij MW, Ikram MA, Wielopolski PA, et al. Cerebral microbleeds: accelerated 3D T2*-weighted GRE MR imaging versus conventional 2D T2*-weighted GRE MR imaging for detection. Radiology 2008; 248: 272–77 [DOI] [PubMed] [Google Scholar]
- 5. Conijn MM, Geerlings MI, Biessels GJ, et al. Cerebral microbleeds on MR imaging: Comparison between 1.5 and 7T. AJNR Am J Neuroradiol 2011; 32: 1043–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goos JD, van der Flier WM, Knol DL, et al. Clinical relevance of improved microbleed detection by susceptibility-weighted magnetic resonance imaging. Stroke 2011; 42: 1894–900 [DOI] [PubMed] [Google Scholar]
- 7. Cordonnier C, Potter GM, Jackson CA, et al. Improving interrater agreement about brain microbleeds: Development of the brain observer microbleed scale (BOMBS). Stroke 2009; 40: 94–99 [DOI] [PubMed] [Google Scholar]
- 8. Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol 2009; 8: 165–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Poels MM, Ikram MA, van der Lugt A, et al. Incidence of cerebral microbleeds in the general population. Stroke 2011; 42: 656–61 [DOI] [PubMed] [Google Scholar]
- 10. de Boer R, Vrooman HA, van der Lijn F, et al. White matter lesion extension to automatic brain tissue segmentation on MRI. Neuroimage 2009; 45: 1151–61 [DOI] [PubMed] [Google Scholar]
- 11. Poels MM, Vernooij MW, Ikram MA, et al. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam Scan Study. Stroke 2010; 41: S103–06 [DOI] [PubMed] [Google Scholar]
- 12. Longstreth WT, Jr, Dulberg C, Manolio TA, et al. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke 2002; 33: 2376–82 [DOI] [PubMed] [Google Scholar]
- 13. Knopman DS, Penman AD, Catellier DJ, et al. Vascular risk factors and longitudinal changes on brain MRI: the ARIC Study. Neurology 2011; 76: 1879–85 [DOI] [PMC free article] [PubMed] [Google Scholar]