Abstract
Background
Mesenchymal stem cell therapy improves ischemic heart failure via incompletely understood mechanisms. C1q-TNFα related protein-9 (CTRP9) is a novel anti-oxidative cardiokine capable of improving the local microenvironment and cell survival by its c-terminal active globular domain (gCTRP9). The current study attempted to: 1) identify active gCTRP9 c-terminal polypeptides with stem cell protective function; 2) determine whether a lead polypeptide may enable/enhance cortical bone-derived mesenchymal stem cell (CBSC) cardioprotection against post-myocardial infarction (post-MI) remodeling; and 3) define the responsible underlying cellular/molecular mechanisms.
Methods and results
Utilizing I-TASSER structure prediction and 3-D active site modeling, we cloned and purified 3 gCTRP9 fragments (CTRP9-237, CTRP9-277, and CTRP9-281). Their activation of cell salvage kinase was compared against gCTRP9. Among the three fragments, CTRP9-281 (a 45 residue-containing polypeptide) exerted comparable or greater ERK1/2 activation compared to gCTRP9. Treatment with CTRP9-281 or gCTRP9 significantly increased CBSC proliferation and migration, and attenuated oxidative stress-induced CBSC apoptosis. CTRP9-281 and gCTRP9 comparably upregulated SOD2 and SOD3 expression. However, CTRP9-281, not gCTRP9, upregulated FGF2 and VEGFA expression/secretion in an ERK1/2 dependent manner. Administration of gCTRP9 or CTRP9-281 alone attenuated post-MI cardiac dysfunction and improved CBSC retention in the infarcted heart in similar fashion. However, CTRP9-281 exerted greater synergistic effect with CBSC than gCTRP9 related to pro-angiogenic, anti-fibrotic, and anti-remodeling effects. Mechanistically, CTRP9-281 significantly increased SOD2-rich and VEGFA-rich exosome production by CBSC. Exosomes from CTRP9-281 treated CBSC significantly attenuated oxidative stress-induced cardiomyocyte apoptosis in vitro. An exosome generation inhibitor attenuated CTRP9-281 enhancement of CBSC cardioprotection in vivo.
Conclusion
We identified a CTRP9 polypeptide that upregulates SOD2/SOD3 expression and improves CBSC survival/retention, similar to gCTRP9. Moreover, CTRP9-281 stimulates VEGFA-rich exosome production by CBSC, exerting superior pro-angiogenic, anti-fibrotic, and cardioprotective actions.
Keywords: Cardiokine, Cell therapy, Exosome, Pathological cardiac remodeling
Highlights
-
•
CTRP9-281 exerts a comparable effect with gCTRP9 in CBSC anti-oxidant gene expression and promots CBSC survival/retention.
-
•
CTRP9-281 plays superior synergistic role with CBSC in promoting angiogenesis and anti-fibrosis of ischemic heart.
-
•
CTRP9-281 significantly enhances SOD-rich/VEGF-rich exosome producted by CBSC, and resists pathological remodeling post-MI.
1. Introduction
Cardiovascular disease accounts for the greatest proportion of premature mortality and morbidity of any noncommunicable disease. Ischemic heart disease, such as myocardial infarction, is the largest contributor to the epidemic of cardiovascular diseases. Adult mammalian hearts have limited regenerative capacity. A decade's worth of evidence supports transplantation of mesenchymal stem cells (MSC) as a potential therapeutic for ischemic heart failure (IHF) [1,2]. However, clinical trials evaluating the efficacy of MSC in patients with acute myocardial infarction injury or decompensated IHF have been indeterminate [3]. Therapeutic strategies enhancing cell therapy efficacy are in great demand.
The most significant limitation for MSC therapy in IHF remains poor retention/survival of engrafted cells [4,5]. MI creates a harsh microenvironment with increased oxidative stress, limited nutrients, and oxygen dearth, inducing damage and death of delivered cells [6,7]. Overcoming this limitation by either promoting MSC survival or improving the local environment may help make cell therapy a reality for myocardial infarction [8]. The mechanisms underlying MSC activity in injured myocardium have been extensively studied. Although it was once believed that MSCs could generate new cardiac tissue, evidence now demonstrates de novo cardiomyocyte regeneration does not occur after MSC administration. The cardioprotective effects of MSC are attributed to secretion of immunomodulatory, anti-autophagic, anti-apoptotic, and pro-angiogenic factors [[9], [10], [11]]. Molecules capable of improving the MSC cardioprotective secretome may enhance cell therapy efficacy against IHF.
The C1q/TNF-related proteins (CTRPs) are a protein family consisting of fifteen (CTRP1-CTRP15) identified adiponectin paralogs [12]. We and others recently demonstrate that CTRP9 is a cardiokine, as it has the greatest expression level in the adult heart [[13], [14], [15]]. MI significantly inhibits myocardial CTRP9 (both mRNA and protein) expression, and reduces plasma CTRP9 levels [16,17]. CTRP9 is critical in maintaining a healthy local environment in the ischemic heart, enhancing intramyocardial injected adipocyte MSC (ADSC) survival, enabling their cardioprotection [5]. Importantly, the c-terminal globular domain isoform of CTRP9 (gCTRP9, residues 197-333), but not full length CTRP9, activates key cell salvage kinases and functions as the active cardioprotective isoform [13,18]. However, whether there exist c-terminal polypeptides retaining g-CTRP9 activity remains unclear. Producing biologically active proteins is difficult, and optimizing the dosage and route of recombinant protein administration is complex. Designing active CTRP9 polypeptides is a highly desirable strategy in realizing the beneficial effects of CTRP9.
Therefore, the aims of the present study were 1) investigating whether gCTRP9-derived polypeptides may exert similar or better stem cell protective function than gCTRP9; 2) determining whether a lead polypeptide may enable/enhance CBSC cardioprotection against post-MI pathological remodeling, and 3) defining the underlying cellular/molecular mechanisms.
2. Methods
2.1. CBSCs isolation, identification, and in vitro studies
CBSCs were isolated from male enhanced green fluorescent protein transgenic mice (The Jackson Laboratory) or littermate C57BL/6J control mice as previously reported (Supplement Figure IA). Cells were cultured in Dulbecco's Modified Eagle Medium-F12-10% FBS. The passage 2 CBSCs were tested for surface marker expression by flow cytometry (BD LSR Fortessa) and differentiation potential. Passage 2 CBSCs express mesenchymal stem cells markers (such as CD105, Scal-1, C-kit, and CD29), but not hematopoietic lineage marker CD45, or endothelial cell marker CD31 (Supplemental Fig. 1A and 1B). Their adipogenic and osteoblastic potential affirm pluripotency (Supplemental Figure 1C).
2.2. Animal study protocol
All experiments were performed in adherence to the National Institutes of Health Guidelines on the Use of Laboratory Animals and were approved by the Thomas Jefferson University Committee on Animal Care. Permanent MI surgery was performed in adult male C57BL/6J mice by ligating the left anterior descending coronary artery. Immediately after MI, 1 × 105 enhanced green fluorescent protein CBSCs suspended in 25 μL PBS (containing 0.2 mM EDTA, pH = 7.3) were administered via intramyocardial injection to the infarct border zone at 3 different sites. 30 min after MI, vehicle, g-CTRP9 (0.25 μg/g/d), or CTRP9-281 (0.25 μg/g/d) was administrated via osmotic pump (peritoneal implant) for 2 weeks. The number of CBSCs engrafted to the heart 1 and 3 days after transplantation was determined via green fluorescent protein (GFP) immunostain as previously reported [5]. 6 weeks after MI, cardiac function was evaluated via echocardiography followed by strain analysis. Fibrosis was evaluated by Masson's trichrome staining. More detailed materials and methods are presented in the online-only Data Supplement.
2.3. Statistical analysis
Data are reported as mean ± SEM. The Kaplan-Meier survival curves were analyzed by a Gehan-Breslow-Wilcoxon test. For analysis of differences between 2 groups, the unpaired Student t-test was performed. For multiple groups, 1-way ANOVA was performed, followed by Bonferroni post hoc test. For multiple groups over time or for testing the interaction of CTRP9/CBSC therapy on heart function after MI, 2-way ANOVA was performed, followed by Bonferroni post hoc test. P values < 0.05 were considered significant. In this study, n is the number of animals or cell cultures tested.
3. Results
3.1. Identification of a CTRP9 polypeptide activating cellular salvage kinases, improving CBSC function via novel mechanism
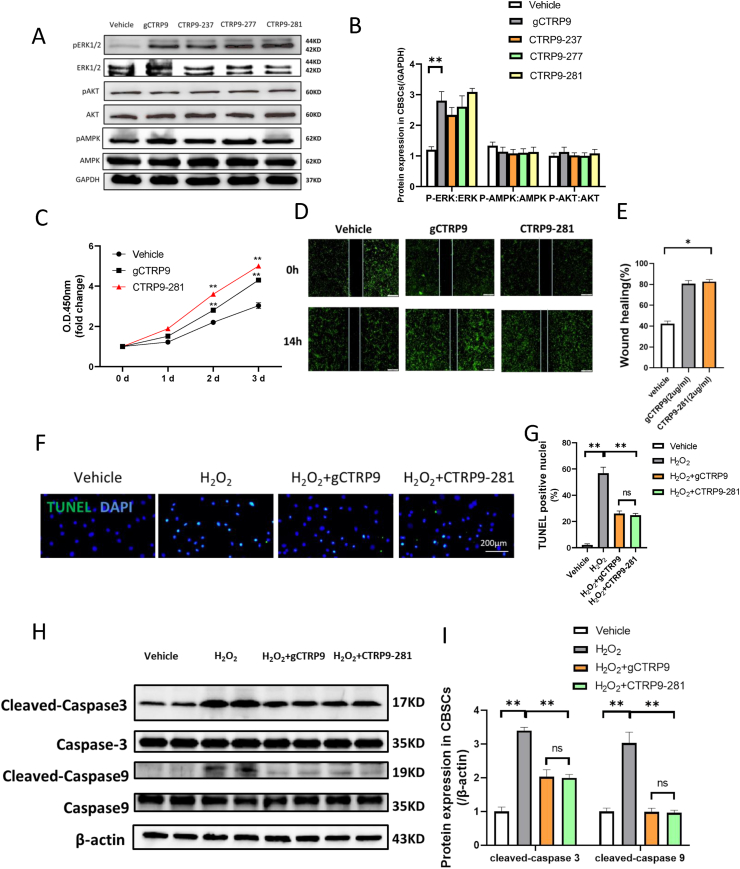
We and others have previously demonstrated that gCTRP9 (residues 197-333) is the active isoform of CTRP9. To maximize CTRP9 translational application, we attempted to identify gCTRP9 polypeptides with capability of activating protective intracellular signaling. Based on the I-TASSER structure prediction and three dimensional modeling of the active site using cofactor and coach [19], we cloned and purified 3 gCTRP9 fragments (CTRP9-237, CTRP9-277, and CTRP9-281, patent pending). In a recent study, we demonstrate that gCTRP9 kinase activation is cell-type dependent [5]. Specifically, gCTRP9 activates multiple cell salvage kinases, including ERK1/2, Akt, and AMPK in adult cardiomyocyte via binding with AdipoR1. However, AdipoR1 expression is low in mesenchymal stem cells and gCTRP9 activates ERK1/2 (not Akt and AMPK) in ADSC via binding with N-cadherin [5]. To determine whether CTRP9 fragments retain gCTRP9 kinase activation profile, we compared the effect of gCTRP9 fragments upon cell salvage kinases against gCTRP9. In H9C2 cells, CTRP9 fragments activated all three cell salvage kinases similar as gCTRP9 (data not shown). More importantly, all three CTRP9 fragments significantly and selectively activated ERK1/2 in CBSC, a property shared with their parent protein, gCTRP9 (Fig. 1A and B). Among these three fragments, CTRP9-281 exerts the best ERK1/2 activation (although not statistically different). Importantly, CTRP9-281 is the shortest fragment and qualifies as a polypeptide (45 aa residue). Finally, no cardiovascular cytotoxic effect (including blood pressure, heart rate, left ventricular function, and hs-cTnT) was observed after bolus administration of CTRP9-281 at 5-50 μg/g (tail vein). CTRP9-281 was therefore our prime molecule of investigation concerning CBSC function and synergistic cardioprotection. We have recently demonstrated that gCTRP9 significantly improves ADSC function. To determine whether CTRP-281 (a polypeptide with better translational value) may improve CBSC (a stem cell type investigated clinically) function, we examined the effect of CTRP9-281 upon CBSC proliferation, migration, and oxidative stress-induced apoptosis. CTRP9-281 increased CBSC proliferation to slightly greater extent compared to gCTRP9 (Cell Counting Kit assay, Fig. 1C). Treatment of CBSC for 14 h with either CTRP9-281 or gCTRP9 significantly enhanced migratory capacity (wound healing assay, Fig. 1D/E). Finally, CTRP9-281 and gCTRP9 exerted a comparable anti-apoptotic (H2O2, 100 μm for 2 h) effect (TUNEL assay, Fig. 1F/G; caspase cleavage, Fig. 1H/I).
Fig. 1.
CTRP9-281, a 45 residues-containing polypeptide, possesses cellular salvage kinase activation property and improves CBSC function comparable as that of gCTRP9. A/B: Effect of gCTRP9, CTRP9-237, CTRP9-277 and CTRP9-281 (2 μg/mL for 15 min) on cell salvage kinase activation in CBSC. n = 4/group, **P < 0.01 vs. vehicle. C: CBSCs proliferation curves (n = 12/group, **P < 0.01 vs. vehicle at indicated time. D/E, Wound healing assay was performed 24 h after vehicle, gCTRP9 or CTRP9-281 treatment. n = 6/group *P < 0.05 vs. vehicle. F-I: CBSCs apoptosis was determined by TUNEL staining and caspase cleavage. CBSCs were treated with 2 μg/mL gCTRP9 or 2 μg/mL CTRP9-281 for 24h followed by H2O2 for 2 h n = 6/group. *P < 0.05, **P < 0.01 vs. vehicle. DAPI, 4′-6-diamidino-2-phenylindole; gCTRP9, globular domain isoform of C1q/tumor necrosis factor–related protein-9; CTRP9-281, 45 residues-containing polypeptides of gCTRP9; O.D., optical density; and TUNEL, transferase-mediated dUTP nick-end labeling.
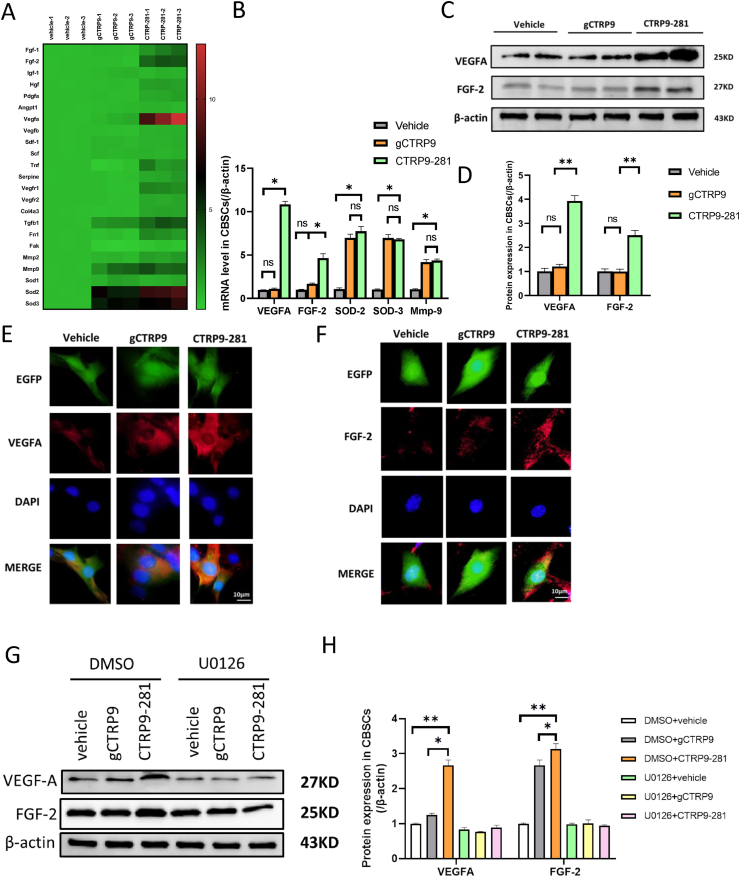
The aforementioned results demonstrate CTRP9-281 exerts similar or greater CBSC protection compared to gCTRP9. We next investigated whether CTRP9-281 exerts its CBSC protective effect by similar or distinctive mechanisms compared to gCTRP9. 23 mRNAs known to involved in cell proliferation, migration, and survival were determined by qT-PCR. Consistent with our recent report, gCTRP9 altered multiple genes, with SOD2, SOD3, and Mmp9 being the three genes most significantly upregulated (Fig. 2A and B). Similar to gCTRP9, CTRP9-281 upregulated these three anti-oxidative/pro-migratory genes (Fig. 2A and B). Interestingly, we observed that CTRP9-281 had a unique property not shared by gCTRP9. CTRP9-281, not gCTRP9, significantly upregulated vascular endothelial growth factor A (Vegfa) and basic fibroblast growth factor (bFgf/Fgf2) (Fig. 2A and B). Western blots analysis (Fig. 2C and D) and immunocellullar fluorescence analysis (Fig. 2E and F) confirmed that CTRP9-281 significantly upregulated VEGFA and FGF2 proteins expression.
Fig. 2.
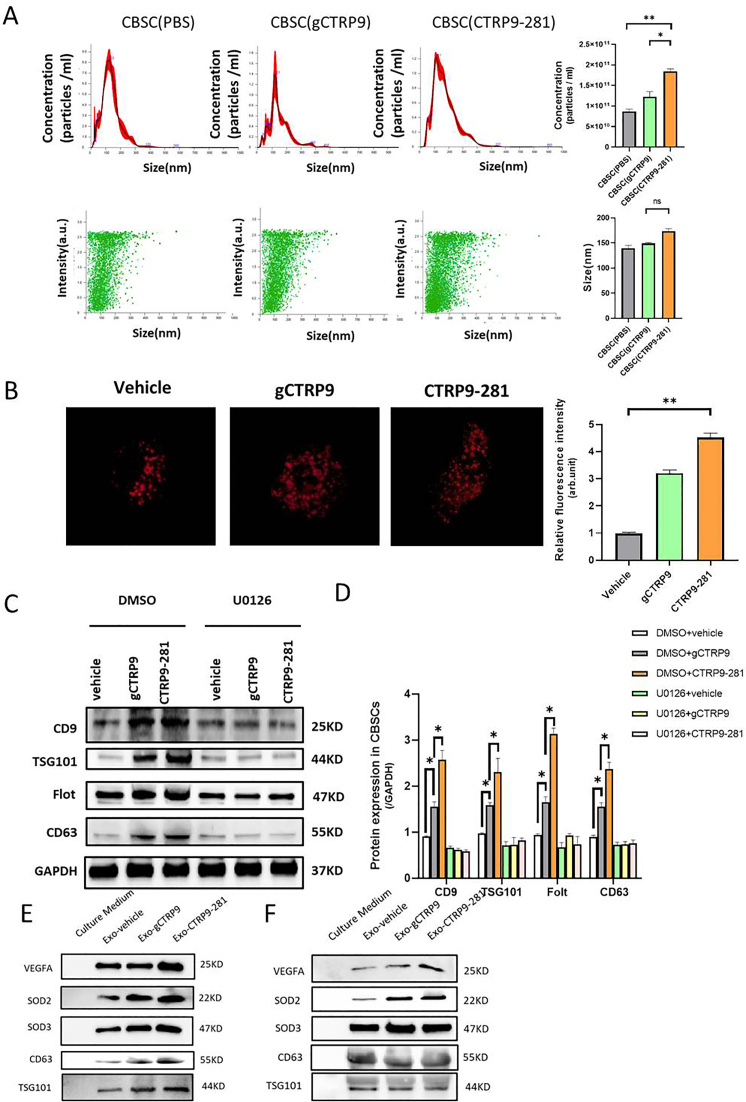
Effect of CTRP9-281 gCTRP9 upon anti-oxidative and pro-angiogenic gene expression. A/B: Gene expression profile of CBSCs treated with vehicle, gCTRP9, or CTRP9-281 (2 μg/ml, 24 h). n = 3/group. *P < 0.05. C/D, Western blots and quantification of protein expression in CBSCs cell lysis. N = 6/group, *P < 0.05. E/F, CBSCs (green) were fixed in vitro and immune-stained against VEGFA and FGF-2 (red). Nuclei are labeled with 4‘,6-diamidino-2-phenylindole (blue) and scale bars, 10 μm. G/H: Western blots and quantification of VEGFA and FGF-2 in CBSCs cell lysis demonstrate that U0126 (an ERK1/2 activation inhibitor) blocked CTRP9-281 induced VEGFA and FGF-2 expression (U0126: 10 μM 2 h before CTRP9-281 treatment) n = 4–5/group, *P < 0.05, **P < 0.01. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The results presented in Fig. 1A and B demonstrated that CTRP9-281 significantly activated ERK1/2 in CBSC. To clarify the causative role of ERK1/2 activation in CTRP9-281 gene/protein upregulation, we determined the effect of ERK1/2 inhibitor upon CTRP9-281 upregulation of pro-angiogenic proteins. ERK1/2 inhibition with U0126 (10 μM, 2 h before CTRP9-281 treatment) virtually abolished the effect of CTRP9-281 upon pro-angiogenic protein expression (Fig. 2G and H).
Taken together, these results demonstrate that CTRP9-281 is a biologically active polypeptide capable of upregulating anti-oxidant/pro-angiogenic gene expression in an ERK1/2-dependent fashion, significantly improving CBSC function and survival.
3.2. CTRP9-281 enhances CBSC retention, promotes angiogenesis, and exerts greater synergistic cardioprotection
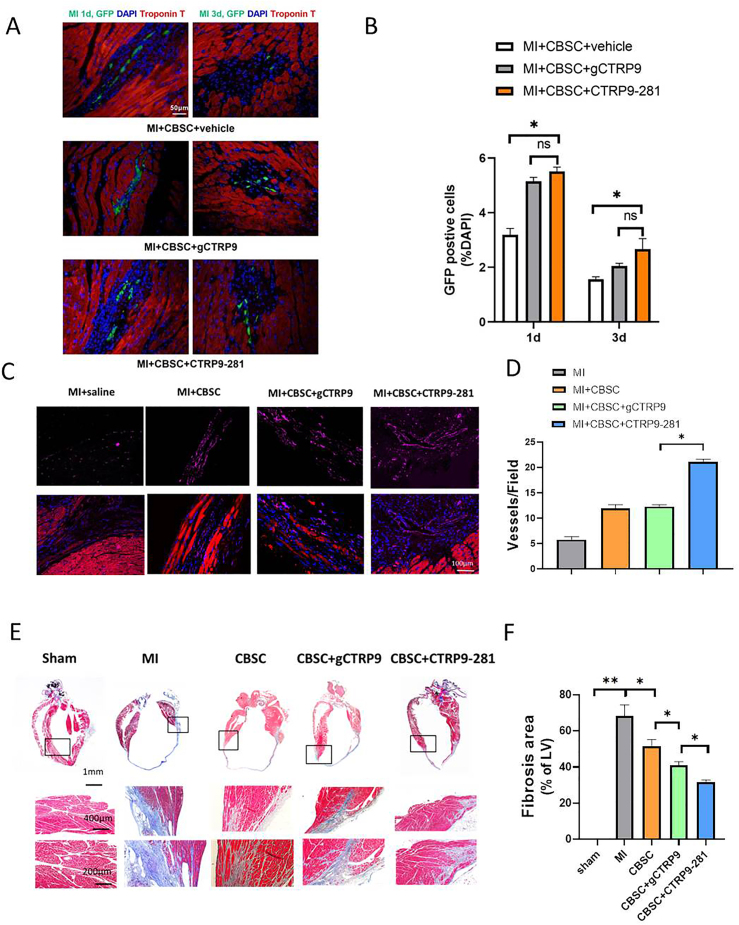
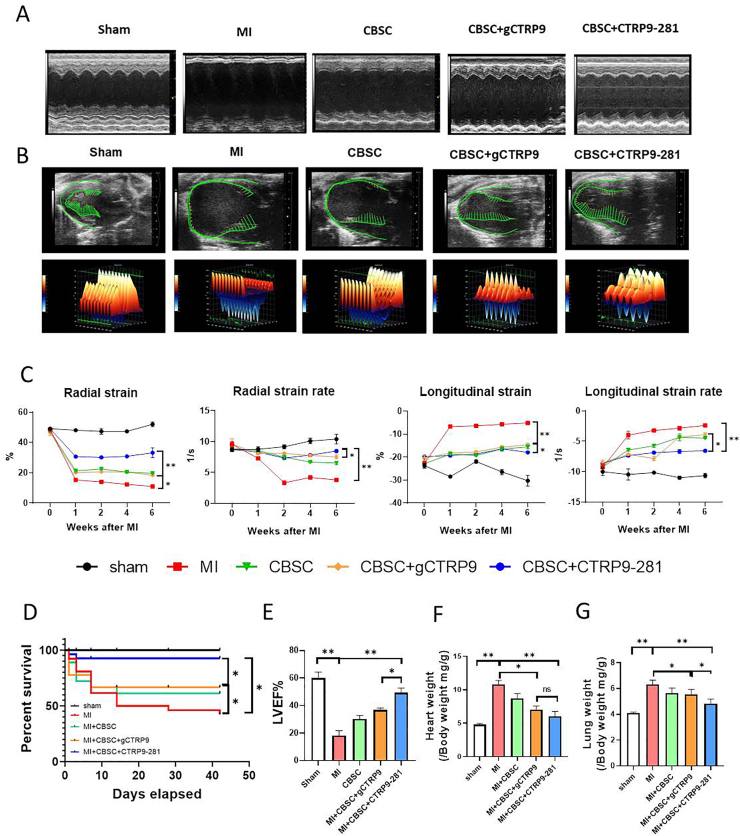
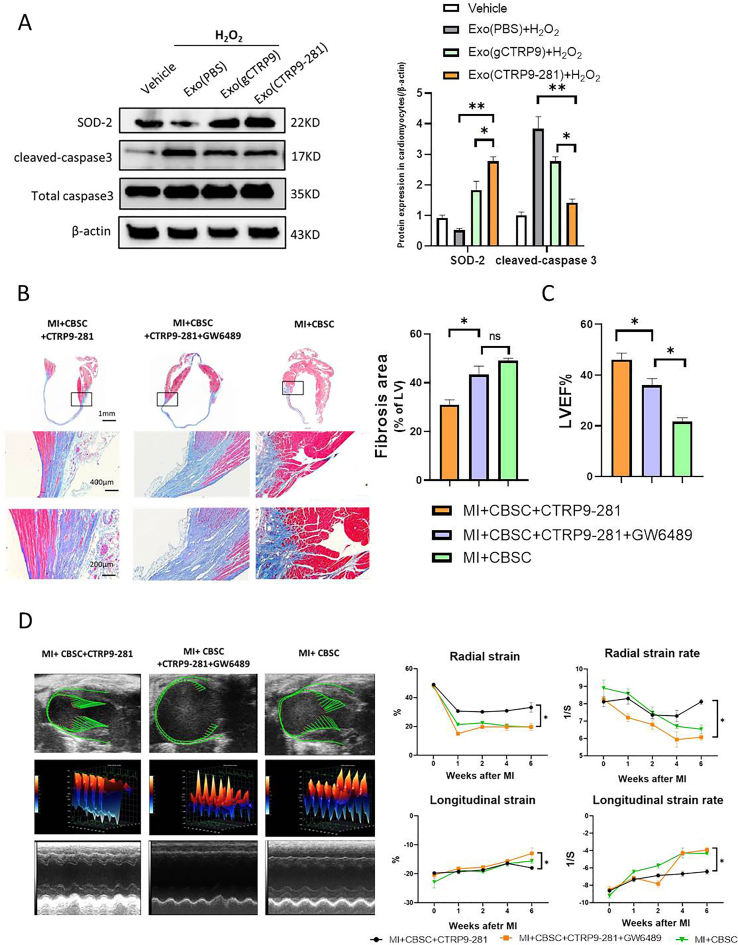
Our pilot experiments demonstrated that administration of gCTRP9 and CTRP9-281 alone (without CBSC) had similar protective effect (Supplement Figure 2). Having demonstrated that CTRP9-281 stimulates VEGFA/FGF2 release by CBSC (in addition to a comparable effect on anti-oxidant molecular expression shared with gCTRP9), we next investigated whether CTRP9-281 may enhance CBSC cardioprotection to greater degree than gCTRP9. First, the effect of CTRP9-281 and gCTRP9 upon CBSC retention, pro-angiogenic, and anti-fibrotic effects were determined. CTRP9-281 increases CBSC retention to similar extent as gCTRP9 (Fig. 3A and B). However, CTRP9-281 promoted CBSC angiogenic (Fig. 3C/D) and anti-fibrotic effects (Fig. 3E and F) significantly greater than gCTRP9. Second, to determine whether the enhanced pro-angiogenic and anti-fibrotic effects result in increased cardioprotection, the effect of CTRP9-281 and gCTRP9 upon CBSC cardiac function was determined by echocardiography followed by strain analysis. Consistent with our previous report utilizing ADSC, CBSC + gCTRP9 significantly improved cardiac function, as evidenced by greater improvement of radial strain and longitudinal strain (Fig. 4A–C). Importantly, CBSC + CTRP9-281 improved cardiac function to greater degree than gCTRP9 (Fig. 4A–C). Third, we determined the impact of CTRP9-281 and gCTRP9 upon CBSC-mediated survival and remodeling effects. Compared to vehicle treated animals, CBSC + gCTRP9 treatment significantly improved survival rate (Fig. 4D), increased LVEF (Fig. 4E), reduced heart weight/body weight ratio (Fig. 4F), and decreased lung weight/body weight ratio (Fig. 4G). More importantly, CBSC + CTRP9-281 treatment markedly improved survival rate, increased LVEF, and reduced lung weight/body weight ratio, to greater extent than CBSC + gCTRP9 (P < 0.05, Fig. 4A-E/G). CBSC + CTRP9-281 treatment reduced heart weight/body weight ratio to greater extent than CBSC + gCTRP9 (although not statistically significant, Fig. 4F). Collectively, these results demonstrate CTRP9-281 has greater synergistic cardioprotective effect with CBSC than gCTRP9, likely due to its VEGF production/angiogenic properties.
Fig. 3.
Effect of CTRP9-281 and gCTRP9 upon CBSC retention, angiogenesis, and fibrosis after MI. A/B: CTRP9-281 and gCTRP9 comparably increased CBSCs survival in peri-infarct area 1 and 3 days after MI. EGFP CBSCs in the peri-infarct area was determined by the number of GFP positive cells per total nuclei (n = 20 from 4 mice). *P < 0.05; NS: not significant. C/D, CTRP9-281 plus CBSCs-treated animals have increased von Willebrand Factor + blood vessels near the infarct border zone by 6 weeks post-MI. Slides from animals receiving MI + vehicle, MI + CBSC or MI + CBSC + gCTRP9 or MI + CBSC + CTRP9-281 injection were stained for von Willebrand factor (purple) and α-sarcomeric actin (red). Nuclei are labeled with DAPI (blue) (n = 30 from 6 mice). *P < 0.05. E/F: Masson's trichrome staining revealed that the cardiac fibrotic area was significantly reduced by CBSC + CTRP9-281administration compared with MI group and CBSC + gCTRP9+. n = 6–9/group, *P < 0.05. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 4.
CBSC + CTRP9-281 exerts greater synergetic cardioprotection superior to CBSC + gCTRP9. A/B: Representative echocardiographic images (A = M mode, B=B mode) 6 weeks after MI. Three-dimensional regional wall strain diagrams showing contraction (orange/positive values) or relaxation (blue/negative values) of 3 consecutive cardiac cycles. C: Strain analysis results of long-axis B-mode images. n = 10–12/group. *P < 0.05, **P < 0.01. D: Survival curves (n = 12/group for sham and 27-45/group for MI). *P < 0.05, **P < 0.01. E: Left ventricular ejection fraction (LVEF), n = 10–18/group**P < 0.01. F: Heart weight/body weight ratio (HW/BW). *P < 0.05, **P < 0.01. G: Lung weight/body weight ratio (LW/BW), n = 10–18. *P < 0.05, **P < 0.01. Survival curves were analyzed by Gehan-Breslow-Wilcoxon test. LVEF were analyzed with 2-way ANOVA followed by a Bonferroni post hoc test. Other data were analyzed with 1-way ANOVA followed by Bonferroni post hoc test. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3. CTRP9-281 increases SOD2/VEGFA-enriched exosome biogenesis by CBSC, contributing to its cardioprotective action
Our results demonstrate CTRP9-281 is uniquely pro-angiogenic, contributing to its synergistic protective effect with CBSC. Considerable evidence exists that cancer cells promote angiogenesis and tumor growth by releasing VEGF-rich exosomes [20]. To determine whether exosomes are involved in CTRP9-281-enhanced CBSC angiogenesis and cardioprotection, several experiments were performed. First, the effect of gCTRP9 and CTRP9-281 upon CBSC (1 × 106/well) exosome production was determined by Nanosight300 and Red fluorescence labeled-HRS (hepatocyte growth factor-regulated tyrosine kinase substrate, a multivesicular body marker). CTRP9-281 increases exosome numbers to greater extent than gCTRP9 (Fig. 5A and B). Neither gCTRP9 nor CTRP9-281 significantly altered exosome size (Fig. 5A). Second, previous studies demonstrate that ERK1/2 regulates exosome biogenesis [21]. We therefore investigated the effect of U0126 (10 mM, 2 h before gCTRP9 or CTRP9-281 treatment) upon CBSC exosome production by surrogate exosome markers, including CD9, TSG101, Flot, and CD63. Although CTRP9-281 stimulated exosomes greater than gCTRP9, pre-treatment with U0126 equally blocked such effects (Fig. 5C/D). Third, to determine whether VEGFA is carried by CBSC-derived exosomes (as observed in cancer cells [20]), exosomes were isolated from vehicle-, gCTRP9-, or CTRP9-281-treated CBSC. Both gCTRP9 and CTRP9-281 significantly increased exosomes containing SOD2/SOD3. CTRP9-281, but not gCTRP9, significantly increased VEGFA levels in CBSC-derived exosomes (Fig. 5E/F).
Fig. 5.
CTRP9-281 increases VEGF-enriched exosome biogenesis by CBSC. A: Nanosight test the number and size distribution of exosomes derived from the CBSCs after PBS, gCTRP9 (2 μg/ml) and CTRP9-281 (2 μg/ml) treatment for 24 h n = 10–12/group, *P < 0.05, **P < 0.01. B: gCTRP9 and CTRP9-281 increase the exosome biogenesis as evidenced by increased multivesicular body (MVBs) formation. CBSC were transfected with pCS2 hepatocyte growth factor-regulated tyrosine kinase substrate (HRS)-RFP plasmid. Cells were treated with vehicle, gCTRP9 or CTRP9-281 for 24 h. The HRS-RFP positive MVBs were significantly increased in CBSCs treatment with gCTRP9 and CTRP9-281 (n ≥ 5, One-Way ANOVA, **p < 0.01). C/D: Western blot showed the Exosome marker protein expression in CBSC after gCTRP9 (2 μg/ml) and CTRP9-281(2 μg/ml) treatment for 24 h. U0126 (an ERK1/2 activation inhibitor) blocked CTRP9-281 and gCTRP9-induced exosome biogenesis (U0126: 10 μM 2 h before CTRP9-281 treatment; n = 4–5/group). E: Western blot analysis of VEGFA, SOD2 and SOD3 expression in exosomes isolated from vehicle, gCTRP9 and CTRP9-281 treated CBSC (1 × 106/well). n = 8/group, *P < 0.05 vs. vehicle. F. Western blot analysis of VEGFA, SOD2 and SOD3 expression in equal number of exosomes. n = 6/group.
In a final attempt to determine the role of exosomes in CTRP9-281-mediated enhancement of CBSC cardioprotection, two additional experiments were performed. First, adult cardiomyocytes were treated with exosomes isolated from vehicle, gCTRP9, or CTRP9-281 treated CBSC. Their effect upon H2O2 (100 μM for 2 h) induced apoptosis was determined. Exosomes isolated from CTRP9-281 treated CBSC exerted superior anti-apoptotic effect compared to that from gCTRP9 treated CBSC (Fig. 6A). Second, CBSC was pre-treated with GW4869 (an exosome biogenesis inhibitor) before intramyocardial injection. GW4869 pre-treatment significantly attenuated CBSC + CTRP9-281 cardioprotection, as determined by cardiac fibrosis (Fig. 6B) and function (Fig. 6C and D). Taken together, these results provide evidence that exosomes derived from CTRP9-281 pre-treated CBSCs contribute to CBSC cardiac protection.
Fig. 6.
Exosomes mediate gCTRP9/CTRP9-281 enhancement of CBSC cardioprotection. A: Western blots and quantification protein expression utilizing adult mouse cardiomyocytes cell lysis. Cardiomyocytes were treated with exosomes isolated from gCTRP9 or CTRP9-281 treated CBSCs for 24h followed by H2O2 treatment for 2 h *P < 0.05, **P < 0.01. B-D: CBSC were pre-treated with neutral sphingomyelinase-2 (nSMase2) inhibitor GW4869 (2 μg/ml) before their intramyocardial injection. GW4869 attenuated the synergistic anti-fibrotic (B) and cardiac functional improvement (C/D) effect of CTRP9-281 and CBSC. n = 6–10/group, *P < 0.05.
4. Discussion
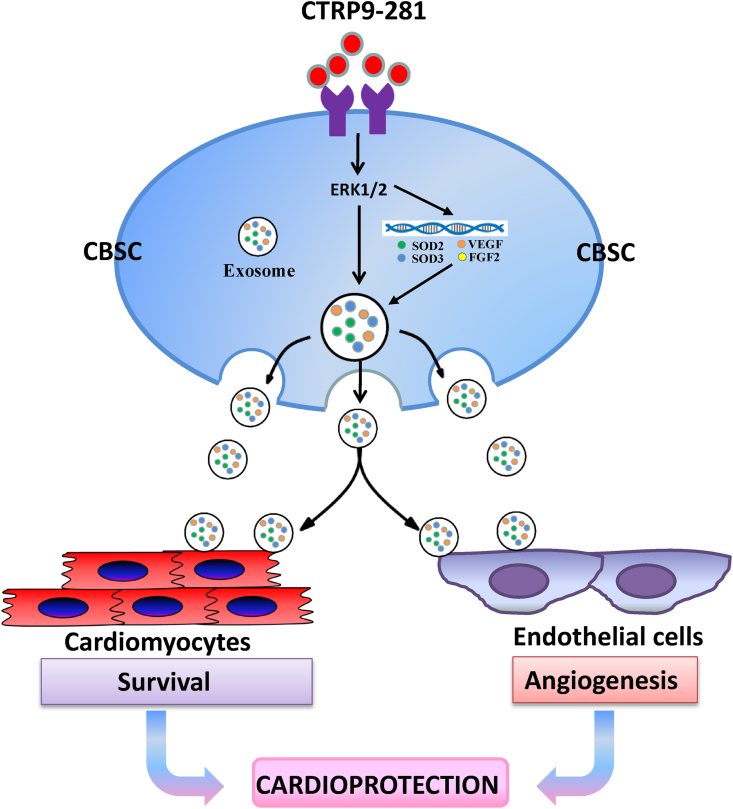
Three novel observations are notable in the current study. First, we identified the first CTRP9 c-terminal polypeptide (CTRP9-281) possessing similar or greater activity than the native cleaved c-terminal globular domain of CTRP9 (gCTRP9) in improving CBSC function. Second, we provided the first in vivo evidence that CTRP9-281 in concert with CBSC exerts more synergistic cardioprotective effect than gCTRP9 plus CBSC, likely due to CTRP9-281's unique promotion of CBSC VEGFA production and resultant angiogenesis. Third, we demonstrated that exosomes play a critical role in mediating CBSC-endothelial cell and CBSC-cardiomyocyte communications, protecting the heart against post-MI pathologic remolding (Fig. 7).
Fig. 7.
Graphic illustration of synergistic cardioprotection of CTRP9-281 plus CBSC and its underlying molecular mechanisms.
CTRP9 was initially discovered in adipocytes during efforts to identify paralogs of adiponectin, an adipocyte-specific cytokine most extensively investigated for its metabolic and cardioprotective actions [22,23]. However, we and others recently demonstrate that CTRP9 is a cardiokine, rather than an adipokine [14,15]. The adult heart expresses the highest levels of CTRP9 of any organ, and CTRP9 is central in maintaining local microenvironmental homeostasis. A recent study of 176 HF patients with 3-year follow-up demonstrates the association between reduced plasma CTRP9 levels and increased mortality [24]. Experimental studies consistently demonstrate that plasma CTRP9 level is significantly reduced in post-MI animals [[25], [26], [27], [28], [29], [30]]. CTRP9KO significantly exacerbates post-MI pathological remolding [5]. Conversely, CTRP9 supplementation attenuates acute ischemia/reperfusion injury, post-MI pathologic ventricular remodeling, and IHF [14,27,28]. Moreover, our recent study demonstrates that CTRP9 circulates in the plasma primarily in the C-terminal globular domain isoform (gCTRP9) [31]. More importantly, gCTRP9, but not full length CTRP9, activates key cardiac survival kinases, underlining the biologic importance of proteolytic cleavage of CTRP931. Finally, we recently demonstrate that CTRP9 is critical in maintaining a healthy local environment in the ischemic heart, enhancing survival of intramyocardial injected stem cells, enabling their cardioprotection [5]. Collectively, strong and consistent evidence from clinical observations and animal studies supports CTRP9 as a cardioprotective cardiokine. However, the translational application of gCTRP9 is limited due to difficulties in producing biologically active protein and optimizing recombinant protein administration dosage and route. Utilizing I-TASSER structure prediction and 3-D active site modeling followed by functional assays, we discovered a CTRP9 C-terminal polypeptide retained biological activity similar to gCTRP9. Similar to gCTRP9, CTRP9-281 upregulates antioxidative molecules. additionally, CTRP9-281 upregulates pro-angiogenic gene expression, significantly improving CBSC function and survival.
Stem cell therapy for the repair of damaged myocardium has evolved into a promising treatment for ischemic heart disease. MSC-based therapy, originating from bone marrow, adipose tissue, or umbilical cord cells, continues to gain consent and appeal because of the large body of preclinical evidence supporting greater paracrine cardioreparative potential. However, poor survival of donated stem cells and failure of stem cell engraftment in the damaged organ pose significant challenges. Increased understanding of the mechanisms enhancing stem cell migration and survival in the microenvironment of the injured myocardium is imperative to improve both cell repair capacity and therapeutic application strategies. We recently demonstrate that CTRP9 is critical in maintaining a healthy microenvironment, facilitating ADSC engraftment in infarcted myocardial tissue and enhancing ADSC cardioprotective efficacy. Specifically, CTRP9KO markedly impaired ADSC survival/retention, whereas gCTRP9 supplementation improves ADSC survival/retention, protecting against post-MI remodeling [5]. In the present study, we demonstrated that although CTRP9-281 has similar potency to gCTRP9 in improving CBSC survival and retention, CTRP9-281 has greater synergistic protective effect with CBSC than gCTRP9. Mechanistic investigation demonstrates that CTRP9-281 stimulation of VEGFA production by CBSC and the resultant pro-angiogenic effect is likely responsible for the augmented cardioprotective function of CTRP9-281.
Although it was once believed that mesenchymal stem cells could generate new cardiac tissue, evidence now suggests that mesenchymal stem cell therapy releases cardioprotective factors which activate endogenous pathways, facilitating myocardial repair-not de novo cardiomyocyte generation [32]. Recent studies demonstrate these factors may cluster into extracellular vesicles, particularly exosomes, which transfer proteins, microRNAs, and lipids to cardiomyocytes, mediating cardioprotection [33,34]. Unfortunately, the reparative capacity of mesenchymal stem cells significantly declines post-MI, as these cells from post-MI patients possess decreased cardioprotective capability [[35], [36], [37]]. Strategies capable of improving the reparative secretome will restore mesenchymal stem cell cardioreparative capability. Moreover, defining the paracrine mechanisms mediating the beneficial effects of mesenchymal stem cell therapy may lead to clinically attractive cell-free therapeutic options. Our current study demonstrated that treatment of CBSC with CTRP9-281 significantly increases SOD-rich and VEGFA-rich exosome production. Treatment with exosomes isolated from CTRP9-281 pre-treated CBSC significantly attenuated oxidative stress-induced cardiomyocyte death. Moreover, pre-treatment of CBSC with exosome biogenesis inhibitor abolished the synergistic effect of CBSC and CTRP9-281. These results are consistent with the notion that cancer cells promote angiogenesis and tumor growth via VEGF-rich exosomes [20].
It should be noted that although both gCTRP9 and CTRP9-281 significantly activate ERK1/2, CTRP-281, not gCTRP9, upregulates Vegfa and Fgf2 expression. Mechanisms responsible for this differential signaling effect are likely complex and requires extensive molecular/cellular/in vivo investigations. One possible explanation is that additional signaling steps are present between ERK1/2 activation and Vegfa and Fgf2 expression. CTRP9-281, not gCTRP9, may activate the molecules required for ERK1/2-mediated Vegfa and Fgf2 expression. This possibility will be investigated in our future study.
In summary, we identified the first CTRP9 c-terminal polypide (CTRP9-281) with strong biological activity. It shares the anti-oxidant/cell survival effects with gCTRP9, promoting CBCS retention after intramyocardial injection. Additionally, CTRP9-281 promotes VEGFA-rich exosome production from CBSC, and exerts augmented angiogenic and cardioprotective effect when co-administered with CBSC in the infarcted heart. Collectively, these results demonstrate that CTRP9-281 may have important translational value in mesenchymal stem cell therapy against ischemic cardiac injury.
Funding sources
This work was supported by awards from the National Institutes of Health (HL-96686, X. Ma/Y. Wang, MPI; HL-123404, X. Ma) and the American Diabetes Association (1-17-IBS-297, Y. Wang).
Declaration of competing interest
The authors declare no competing interests, financial or otherwise.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2021.101929.
Contributor Information
Xin-Liang Ma, Email: xin.ma@jefferson.edu.
Yajing Wang, Email: yajing.wang@jefferson.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Karantalis V., DiFede D.L., Gerstenblith G., Pham S., Symes J., Zambrano J.P., Fishman J., Pattany P., McNiece I., Conte J., Schulman S., Wu K., Shah A., Breton E., Davis-Sproul J., Schwarz R., Feigenbaum G., Mushtaq M., Suncion V.Y., Lardo A.C., Borrello I., Mendizabal A., Karas T.Z., Byrnes J., Lowery M., Heldman A.W., Hare J.M. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: the Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) trial. Circ. Res. 2014;114:1302–1310. doi: 10.1161/CIRCRESAHA.114.303180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bortolotti F., Ruozi G., Falcione A., Doimo S., Dal Ferro M., Lesizza P., Zentilin L., Banks L., Zacchigna S., Giacca M. In vivo functional selection identifies cardiotrophin-1 as a cardiac engraftment factor for mesenchymal stromal cells. Circulation. 2017;136:1509–1524. doi: 10.1161/CIRCULATIONAHA.117.029003. [DOI] [PubMed] [Google Scholar]
- 3.Vagnozzi R.J., Maillet M., Sargent M.A., Khalil H., Johansen A.K.Z., Schwanekamp J.A., York A.J., Huang V., Nahrendorf M., Sadayappan S., Molkentin J.D. An acute immune response underlies the benefit of cardiac stem cell therapy. Nature. 2020;577:405–409. doi: 10.1038/s41586-019-1802-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang W.E., Yang D., Li L., Wang W., Peng Y., Chen C., Chen P., Xia X., Wang H., Jiang J., Liao Q., Li Y., Xie G., Huang H., Guo Y., Ye L., Duan D.D., Chen X., Houser S.R., Zeng C. Prolyl hydroxylase domain protein 2 silencing enhances the survival and paracrine function of transplanted adipose-derived stem cells in infarcted myocardium. Circ. Res. 2013;113:288–300. doi: 10.1161/CIRCRESAHA.113.300929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan W., Guo Y., Tao L., Lau W.B., Gan L., Yan Z., Guo R., Gao E., Wong G.W., Koch W.L., Wang Y., Ma X.L. C1q/Tumor necrosis factor-related protein-9 regulates the fate of implanted mesenchymal stem cells and mobilizes their protective effects against ischemic heart injury via multiple novel signaling pathways. Circulation. 2017;136:2162–2177. doi: 10.1161/CIRCULATIONAHA.117.029557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tu C., Mezynski R., Wu J.C. Improving the engraftment and integration of cell transplantation for cardiac regeneration. Cardiovasc. Res. 2019:cvz237. doi: 10.1093/cvr/cvz237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu X., Xu Y., Zhong Z., Wu Y., Zhao J., Wang Y., Cheng H., Kong M., Zhang F., Chen Q., Sun J., Li Q., Jin J., Li Q., Chen L., Wang C., Zhan H., Fan Y., Yang Q., Yu L., Wu R., Liang J., Zhu J., Wang Y., Jin Y., Lin Y., Yang F., Jia L., Zhu W., Chen J., Yu H., Zhang J., Wang J. A large-scale investigation of hypoxia-preconditioned allogeneic mesenchymal stem cells for myocardial repair in nonhuman primates: paracrine activity without remuscularization. Circ. Res. 2016;118:970–983. doi: 10.1161/CIRCRESAHA.115.307516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor D.A., Chandler A.M., Gobin A.S., Sampaio L.C. Maximizing cardiac repair: should we focus on the cells or on the matrix? Circ. Res. 2017;120:30–32. doi: 10.1161/CIRCRESAHA.116.309959. [DOI] [PubMed] [Google Scholar]
- 9.Wang N., Chen C., Yang D., Liao Q., Luo H., Wang X., Zhou F., Yang X., Yang J., Zeng C., Wang W.E. Mesenchymal stem cells-derived extracellular vesicles, via miR-210, improve infarcted cardiac function by promotion of angiogenesis. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2017;1863:2085–2092. doi: 10.1016/j.bbadis.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 10.Xiao C., Wang K., Xu Y., Hu H., Zhang N., Wang Y., Zhong Z., Zhao J., Li Q., Zhu D., Ke C., Zhong S., Wu X., Yu H., Zhu W., Chen J., Zhang J., Wang J., Hu X. Transplanted mesenchymal stem cells reduce autophagic flux in infarcted hearts via the exosomal transfer of miR-125b. Circ. Res. 2018;123:564–578. doi: 10.1161/CIRCRESAHA.118.312758. [DOI] [PubMed] [Google Scholar]
- 11.Naftali-Shani N., Levin-Kotler L.P., Palevski D., Amit U., Kain D., Landa N., Hochhauser E., Leor J. Left ventricular dysfunction switches mesenchymal stromal cells toward an inflammatory phenotype and impairs their reparative properties via toll-like receptor-4. Circulation. 2017;135:2271–2287. doi: 10.1161/CIRCULATIONAHA.116.023527. [DOI] [PubMed] [Google Scholar]
- 12.Ouchi N., Walsh K. Cardiovascular and metabolic regulation by the adiponectin/C1q/tumor necrosis factor-related protein family of proteins. Circulation. 2012;125:3066–3068. doi: 10.1161/CIRCULATIONAHA.112.114181. [DOI] [PubMed] [Google Scholar]
- 13.Yuan Y., Lau W.B., Su H., Sun Y., Yi W., Du Y., Christopher T., Lopez B., Wang Y., Ma X.L. C1q-TNF-related protein-9, a novel cardioprotetcive cardiokine, requires proteolytic cleavage to generate a biologically active globular domain isoform. Am. J. Physiol. Endocrinol. Metab. 2015;308:E891–E898. doi: 10.1152/ajpendo.00450.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su H., Yuan Y., Wang X.M., Lau W.B., Wang Y., Wang X., Gao E., Koch W.J., Ma X.L. Inhibition of CTRP9, a novel and cardiac-abundantly expressed cell survival molecule, by TNFalpha-initiated oxidative signaling contributes to exacerbated cardiac injury in diabetic mice. Basic Res. Cardiol. 2013;108:315–326. doi: 10.1007/s00395-012-0315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Appari M., Breitbart A., Brandes F., Szaroszyk M., Froese N., Korf-Klingebiel M., Mohammadi M.M., Grund A., Scharf G.M., Wang H., Zwadlo C., Fraccarollo D., Schrameck U., Nemer M., Wong G.W., Katus H.A., Wollert K.C., Muller O.J., Bauersachs J., Heineke J. C1q-TNF-Related protein-9 promotes cardiac hypertrophy and failure. Circ. Res. 2017;120:66–77. doi: 10.1161/CIRCRESAHA.116.309398. [DOI] [PubMed] [Google Scholar]
- 16.Kambara T., Ohashi K., Shibata R., Ogura Y., Maruyama S., Enomoto T., Uemura Y., Shimizu Y., Yuasa D., Matsuo K., Miyabe M., Kataoka Y., Murohara T., Ouchi N. CTRP9 protein protects against myocardial injury following ischemia-reperfusion through AMP-activated protein kinase (AMPK)-dependent mechanism. J. Biol. Chem. 2012;287:18965–18973. doi: 10.1074/jbc.M112.357939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kambara T., Shibata R., Ohashi K., Matsuo K., Hiramatsu-Ito M., Enomoto T., Yuasa D., Ito M., Hayakawa S., Ogawa H., Aprahamian T., Walsh K., Murohara T., Ouchi N. C1q/Tumor necrosis factor-related protein 9 protects against acute myocardial injury through an adiponectin receptor I-AMPK-Dependent mechanism. Mol. Cell Biol. 2015;35:2173–2185. doi: 10.1128/MCB.01518-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su H., Yuan Y., Wang X.M., Lau W.B., Wang Y., Wang X., Gao E., Koch W.J., Ma X.L. Inhibition of CTRP9, a novel and cardiac-abundantly expressed cell survival molecule, by TNFalpha-initiated oxidative signaling contributes to exacerbated cardiac injury in diabetic mice. Basic Res. Cardiol. 2013;108:315. doi: 10.1007/s00395-012-0315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roy A., Kucukural A., Zhang Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat. Protoc. 2010;5:725–738. doi: 10.1038/nprot.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christianson H.C., Svensson K.J., van Kuppevelt T.H., Li J.P., Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc. Natl. Acad. Sci. U. S. A. 2013;110:17380–17385. doi: 10.1073/pnas.1304266110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Datta A., Kim H., Lal M., McGee L., Johnson A., Moustafa A.A., Jones J.C., Mondal D., Ferrer M., Abdel-Mageed A.B. Manumycin A suppresses exosome biogenesis and secretion via targeted inhibition of Ras/Raf/ERK1/2 signaling and hnRNP H1 in castration-resistant prostate cancer cells. Canc. Lett. 2017;408:73–81. doi: 10.1016/j.canlet.2017.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berg A.H., Combs T.P., Scherer P.E. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol. Metabol. 2002;13:84–89. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- 23.Chandran M., Phillips S.A., Ciaraldi T., Henry R.R. Adiponectin: more than just another fat cell hormone? Diabetes Care. 2003;26:2442–2450. doi: 10.2337/diacare.26.8.2442. [DOI] [PubMed] [Google Scholar]
- 24.Gao C., Zhao S., Lian K., Mi B., Si R., Tan Z., Fu F., Wang S., Wang R., Ma X., Tao L. C1q/TNF-related protein 3 (CTRP3) and 9 (CTRP9) concentrations are decreased in patients with heart failure and are associated with increased morbidity and mortality. BMC Cardiovasc. Disord. 2019;19:139. doi: 10.1186/s12872-019-1117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bai S., Cheng L., Yang Y., Fan C., Zhao D., Qin Z., Feng X., Zhao L., Ma J., Wang X., Yang J., Xu X., Yi D., Yi W. C1q/TNF-Related protein 9 protects diabetic rat heart against ischemia reperfusion injury: role of endoplasmic reticulum stress. Oxid Med Cell Longev. 2016;2016:1902025. doi: 10.1155/2016/1902025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kambara T., Shibata R., Ohashi K., Matsuo K., Hiramatsu-Ito M., Enomoto T., Yuasa D., Ito M., Hayakawa S., Ogawa H., Aprahamian T., Walsh K., Murohara T., Ouchi N. C1q/Tumor necrosis factor-related protein 9 protects against acute myocardial injury through an adiponectin receptor I-AMPK-Dependent mechanism. Mol. Cell Biol. 2015;35:2173–2185. doi: 10.1128/MCB.01518-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun Y., Yi W., Yuan Y., Lau W.B., Yi D., Wang X., Wang Y., Su H., Wang X., Gao E., Koch W.J., Ma X.L. C1q/tumor necrosis factor-related protein-9, a novel adipocyte-derived cytokine, attenuates adverse remodeling in the ischemic mouse heart via protein kinase A activation. Circulation. 2013;128:S113–S120. doi: 10.1161/CIRCULATIONAHA.112.000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kambara T., Ohashi K., Shibata R., Ogura Y., Maruyama S., Enomoto T., Uemura Y., Shimizu Y., Yuasa D., Matsuo K., Miyabe M., Kataoka Y., Murohara T., Ouchi N. CTRP9 protects against myocardial injury following ischemia-reperfusion through AMPK-dependent mechanism. J. Biol. Chem. 2012;287:18965–18973. doi: 10.1074/jbc.M112.357939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peterson J.M., Wei Z., Seldin M.M., Byerly M.S., Aja S., Wong G.W. CTRP9 transgenic mice are protected from diet-induced obesity and metabolic dysfunction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;305:R522–R533. doi: 10.1152/ajpregu.00110.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei Z., Lei X., Petersen P.S., Aja S., Wong G.W. Targeted deletion of C1q/TNF-related protein 9 increases food intake, decreases insulin sensitivity, and promotes hepatic steatosis in mice. Am. J. Physiol. Endocrinol. Metab. 2014;306:E779–E790. doi: 10.1152/ajpendo.00593.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan Y., Lau W.B., Su H., Sun Y., Yi W., Du Y., Christopher T., Lopez B., Wang Y., Ma X.L. C1q-TNF-related protein-9, a novel cardioprotetcive cardiokine, requires proteolytic cleavage to generate a biologically active globular domain isoform. Am. J. Physiol. Endocrinol. Metab. 2015;308:E891–E898. doi: 10.1152/ajpendo.00450.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen P.K., Rhee J.W., Wu J.C. Adult stem cell therapy and heart failure, 2000 to 2016: a systematic review. JAMA Cardiol. 2016;1:831–841. doi: 10.1001/jamacardio.2016.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sluijter J.P.G., Davidson S.M., Boulanger C.M., Buzas E.I., de Kleijn D.P.V., Engel F.B., Giricz Z., Hausenloy D.J., Kishore R., Lecour S., Leor J., Madonna R., Perrino C., Prunier F., Sahoo S., Schiffelers R.M., Schulz R., Van Laake L.W., Ytrehus K., Ferdinandy P. Extracellular vesicles in diagnostics and therapy of the ischaemic heart: position paper from the working group on cellular biology of the heart of the European society of Cardiology. Cardiovasc. Res. 2018;114:19–34. doi: 10.1093/cvr/cvx211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kishore R., Khan M. More than tiny sacks: stem cell exosomes as cell-free modality for cardiac repair. Circ. Res. 2016;118:330–343. doi: 10.1161/CIRCRESAHA.115.307654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cogle C.R., Wise E., Meacham A.M., Zierold C., Traverse J.H., Henry T.D., Perin E.C., Willerson J.T., Ellis S.G., Carlson M., Zhao D.X., Bolli R., Cooke J.P., Anwaruddin S., Bhatnagar A., da Graca Cabreira-Hansen M., Grant M.B., Lai D., Moye L., Ebert R.F., Olson R.E., Sayre S.L., Schulman I.H., Bosse R.C., Scott E.W., Simari R.D., Pepine C.J., Taylor D.A., Cardiovascular Cell Therapy Research N. Detailed analysis of bone marrow from patients with ischemic heart disease and left ventricular dysfunction: BM CD34, CD11b, and clonogenic capacity as biomarkers for clinical outcomes. Circ. Res. 2014;115:867–874. doi: 10.1161/CIRCRESAHA.115.304353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Westenbrink B.D., Voors A.A., de Boer R.A., Schuringa J.J., Klinkenberg T., van der Harst P., Vellenga E., van Veldhuisen D.J., van Gilst W.H. Bone marrow dysfunction in chronic heart failure patients. Eur. J. Heart Fail. 2010;12:676–684. doi: 10.1093/eurjhf/hfq061. [DOI] [PubMed] [Google Scholar]
- 37.Nollet E., Hoymans V.Y., Rodrigus I.R., De Bock D., Dom M., Vanassche B., Van Hoof V.O., Cools N., Van Ackeren K., Wouters K., Vermeulen K., Vrints C.J., Van Craenenbroeck E.M. Bone marrow-derived progenitor cells are functionally impaired in ischemic heart disease. J Cardiovasc Transl Res. 2016;9:266–278. doi: 10.1007/s12265-016-9707-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.