Figure 1.
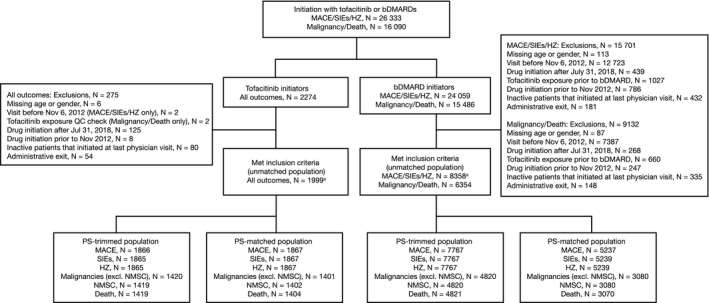
Number of patients eligible for the analysis of major adverse cardiovascular events (MACE), serious infection events (SIEs), herpes zoster (HZ), malignancies (excluding nonmelanoma skin cancer [NMSC]), NMSC, and death. The index date was the initiation date of tofacitinib or a biological disease‐modifying antirheumatic drug (bDMARD); multiple bDMARD initiations were considered separate index events for MACE, SIEs, and HZ, but were not considered separate index events for malignancy/death; propensity scores (PSs) were calculated using baseline patient demographic data (age, sex, marital status, and work status), disease characteristics (duration of rheumatoid arthritis [RA], disease activity [for malignancy/death only: patient pain, patient fatigue, Health Assessment Questionnaire, and EuroQol Five‐Dimensions Health Questionnaire]), comorbidities (history of adverse event of interest [yes versus no; excluding cardiovascular disease–related deaths for MACE and excluding death]), past medication history, and concomitant medications. Venous thromboembolic events were assessed in the unmatched population derived for the analysis of MACE, SIE, and HZ. QC, quality control.
