Figure 2.
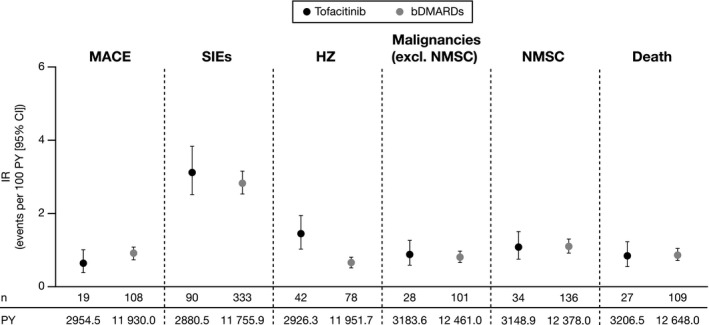
Incidence rates (IRs; number of first events/100 patient‐years [PY]) of outcomes in the propensity score–trimmed population. IRs were based on different definitions of the risk window for outcomes with acute onset (major cardiovascular adverse events [MACE], serious infection events [SIEs], and herpes zoster [HZ]) or latent onset (malignancies and death). Tofacitinib initiators primarily received tofacitinib 5 mg twice daily. bDMARD, biological disease‐modifying antirheumatic drug; CI, confidence interval; NMSC, nonmelanoma skin cancer.
