Figure 4.
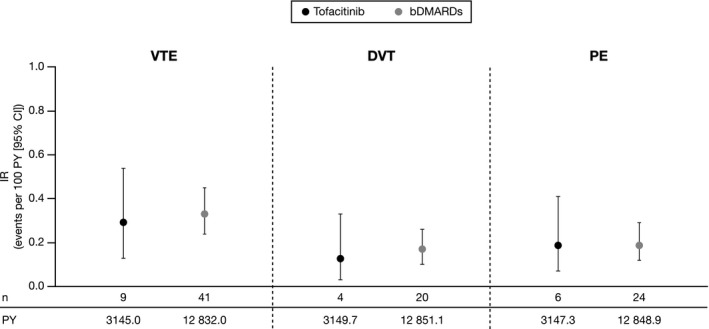
Age‐ and sex‐standardized incidence rates (number of first events/100 patient‐years [PY]) of venous thromboembolic events (VTEs). IRs were based on the definition of the risk window for outcomes with acute onset. Age‐ and sex‐standardized IRs were estimated using direct standardization (tofacitinib population used as standard population); VTE data did not have 80% or more power to detect a hazard ratio (HR) of 2.25 or less between cohorts. Propensity scores were not calculated. Tofacitinib initiators primarily received tofacitinib 5 mg twice daily. bDMARD, biological disease‐modifying antirheumatic drug; CI, confidence interval; DVT, deep vein thrombosis; IR, incidence rate; PE, pulmonary embolism.
