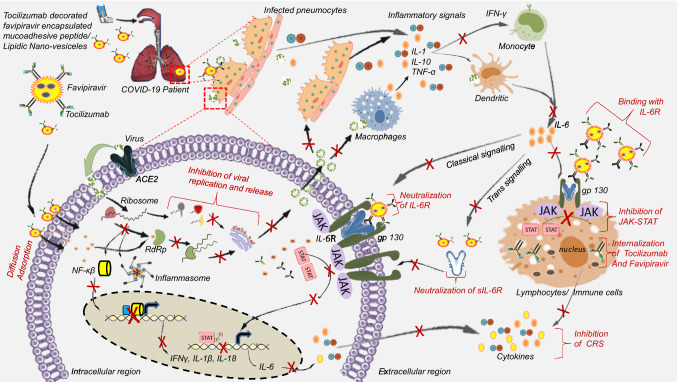
Fig. 1.
Graphical presentation of the proposed hypothesis. During infection, SARS-CoV-2 using spike glycoprotein attaches to the ACE2 receptor on the host cell membrane and enters inside the cell where RNA translates to form RdRp, spike, nucleo-capsid, and other proteins, which later assemble and package through the endoplasmic reticulum and Golgi body and finally get released outside the cell as an infectious virion. Excessive damage to host cells results in the immune cell recruitment phenomenon which eventually leads to cytokine release syndrome. Especially IL-6, which binds to the IL-6R and activates the gp130 medicated JAK/STAT pathway. Favipiravir and Tocilizumab co-delivery using mucoadhesive protein-lipidic nanovesicles administered through pulmonary route by nebulizer will help in two ways: Mucoadhesiveness would help the nanovesicles to have an extended retention time in the lung tissues. Nanovesicles will facilitate pro-antiviral favipiravir to be delivered inside the infected cells where it gets activated by ribosylation and phosphorylation to active favipiravir-RTP, which subsequently will bind the RdRp enzyme and inhibit viral replication. This will cease virion production. Tocilizumab antibody coated onto the nanovesicles would bind to the soluble IL-6R or IL-6R present on the cell membrane, thereby restricting IL-6 binding with the receptors. This will result in the inhibition of JAK/STAT signaling pathway and simultaneously, reduce the inflammatory cascade reaction